Main Text
1 Introduction
Henoch-Schonlein purpura is a systemic vasculitis, and its incidence rate is relatively high in childhood [1,2]. The accumulation of immune complexes produced by allergic purpura on the vessel walls can lead to vasculitis. Focal necrosis of the vessel walls and platelet thrombosis may also occur, which has a serious impact on children's growth, development and quality of life [3,4]. Clinically, Henoch-Schonlein purpura is classified into cutaneous, abdominal, arthritic, renal, and mixed types based on the affected sites. The main manifestations include skin purpura, arthritis, abdominal pain, renal involvement, and melena. Among them, abdominal and some mixed types of Henoch-Schonlein purpura are accompanied by gastrointestinal symptoms such as abdominal pain, vomiting, gastrointestinal bleeding, and intussusception [5-8]. Children with Henoch-Schonlein purpura complicated with gastrointestinal symptoms are often misdiagnosed as other diseases in clinical diagnosis due to the appearance of rash symptoms later than gastrointestinal symptoms. Therefore, finding laboratory testing indicators that can indicate Henoch-Schonlein purpura is beneficial for improving diagnostic accuracy [9,10]. The onset of Henoch-Schonlein purpura in children is mainly related to infections, adverse reactions to food or drugs, and vaccines. Studies [11] have revealed that most children have varying degrees of immune dysfunction, and the pathogenesis and clinical symptoms of Henoch-Schonlein purpura may be associated with the levels of immunoglobulins and lymphocyte subsets in the children's bodies. On this basis, this study compared the diagnostic value of lymphocyte subsets combined with immunoglobulin for different symptoms of Henoch-Schonlein purpura in children, and explored the diagnostic value of lymphocyte subsets combined with immunoglobulin for this disease, hoping to provide reference for the clinical diagnosis of gastrointestinal symptoms in children with Henoch-Schonlein purpura.
2 Materials and methods
2.1 General information
The clinical data of 80 children with Henoch-Schonlein purpura treated in our hospital from December 2017 to December 2022 were retrospectively analyzed. According to the symptoms of Henoch-Schonlein purpura, the children were divided into a complicated group and a simple group. 40 children with Henoch-Schonlein purpura complicated with gastrointestinal symptoms were assigned as the complicated group. 40 cases of purpura simplex with skin purpura as the first symptom were set as the simple group. The study was approved by the Medical Ethics Committee (Lun Approval No. 2401), and all the family members of the children patients signed the informed consent form.
2.2 Inclusion and exclusion criteria
2.2.1 Inclusion criteria
Compliance with the diagnostic criteria for Henoch-Schonlein purpura in the "Evidence-Based Diagnosis and Treatment Recommendations for Children with Henoch-Schonlein purpura" [12]; (2) Age between 3 and 12 years; (3) No previous history of Henoch-Schonlein purpura; (4) No use of corticosteroids or immunosuppressive agents within the past month; (5) Vaccination according to the national immunization schedule, including hepatitis B vaccines.
2.2.1 Inclusion criteria
Acute gastroenteritis, appendicitis, biliary diseases, peptic ulcers, and other gastrointestinal diseases; (2) Severe dysfunction of vital organs such as the heart, liver, or kidneys; (3) Immune dysfunction; (4) Mental disorders with poor treatment compliance; (5) Incomplete clinical data.
2.3 Methods
2 mL samples of fasting venous blood were collected from all children in the morning. The blood was allowed to clot at low temperature for 2 h, and centrifuged at 4000 r/min for 15 min. The upper serum was stored at -30 ℃ for later use. The lymphocyte subsets, including T, B, and natural killer (NK) cells, were detected using the Bricyte-E6 flow cytometer from Shenzhen Mindray Bio-Medical Electronics Co., Ltd., with Mindray's four-color composite antibody as the detection reagent. The levels of immunoglobulins (Ig) A and IgG were measured using the ABBOTT ARCHITECT C16000 biochemical analyzer from Biosino (China), and the IgE level was detected by electrochemiluminescence immunoassay using reagent kits from Roche Diagnostics (Shanghai) Co., Ltd.
2.4 Observational indicators
2.4.1 General data
The gender, age, season of onset, factor, severity of purpura, distribution of purpura, and number of cases with gastrointestinal symptoms were recorded for both groups of children.
2.4.2 Lymphocyte subsets
The counts of T, B, and NK cells, as well as the levels of IgA, IgE, and IgG, were recorded for both groups of children.
2.5 Statistical methods
Statistical analysis was performed using SPSS 20.0. Enumeration data were expressed as percentage (%), and comparisons between the two groups were performed using the X2 test. Measurement data were described as mean ± standard deviation. Comparisons between the two groups were completed with the independent samples t-test. The Spearman method was used to compare the correlation between NK cell count, IgA level, IgE level and IgG level with gastrointestinal symptoms in children with Henoch-Schonlein purpura. The area under the receiver operating characteristic (ROC) curve (AUC) was applied to analyze the diagnostic value of each parameter. p < 0.05 implied statistically significant difference.
3 Results
3.1 General data
The main seasons of onset were autumn and winter. The main causes of the disease were respiratory infections, urinary tract infections, seafood allergies, bronchitis, or mosquito bites. The severity of purpura was determined by the color, distribution range, and presence of other symptoms on the skin. The main distribution sites of skin purpura were the extensor sides of the limbs, lower limbs, and buttocks. The main gastrointestinal symptoms were abdominal pain and hemafecia, with a few cases of gastrointestinal bleeding. There were no statistically significant differences between the two groups of children in terms of gender, age, season of onset, factors, severity of purpura, distribution of skin purpura, or gastrointestinal symptoms (p > 0.05, Table 1), indicating comparability between the groups.
Table 1 Comparison of general information between the two groups (mean ± standard deviation).
| Group | Simple group (n = 40) | Complicated group (n = 40) | t/X2 | p | |
|---|---|---|---|---|---|
| Sex (case) | Male | 18 | 21 | 0.45 | 0.502 |
| Female | 22 | 19 | |||
| Age (year) | 6.15 ± 1.67 | 6.48 ± 1.75 | 0.848 | 0.399 | |
| Season of onset (case) | Spring | 9 | 7 | 0.687 | 0.876 |
| Summer | 7 | 6 | |||
| Autumn | 13 | 13 | |||
| Winter | 11 | 14 | |||
| Factors of disease (case) | Respiratory tract infection | 16 | 15 | 1.34 | 0.855 |
| Urinary tract infection | 8 | 11 | |||
| Seafood allergies | 6 | 7 | |||
| Bronchitis | 6 | 5 | |||
| Mosquito bite | 4 | 2 | |||
| Severity of purpura (case) | Yes | 18 | 17 | 0.051 | 0.822 |
| No | 22 | 23 | |||
| Distribution of purpura (case) | The extensor sides of the limbs | 13 | 15 | 0.497 | 0.78 |
| Lower limbs | 16 | 13 | |||
| Buttocks | 11 | 12 | |||
| Distribution of digestive tract symptoms (case) | Abdominal pain | 9 | 10 | 0.421 | 0.81 |
| Hemafecia | 6 | 4 | |||
| Gastrointestinal bleeding | 1 | 1 | |||
3.2 Lymphocyte subsets
There was no statistically significant difference in the counts of T and B cells between the two groups of children (p > 0.05). The count of NK cells in the complicated group was notably lower than that in the simple group (p < 0.05, Figures 1-3).
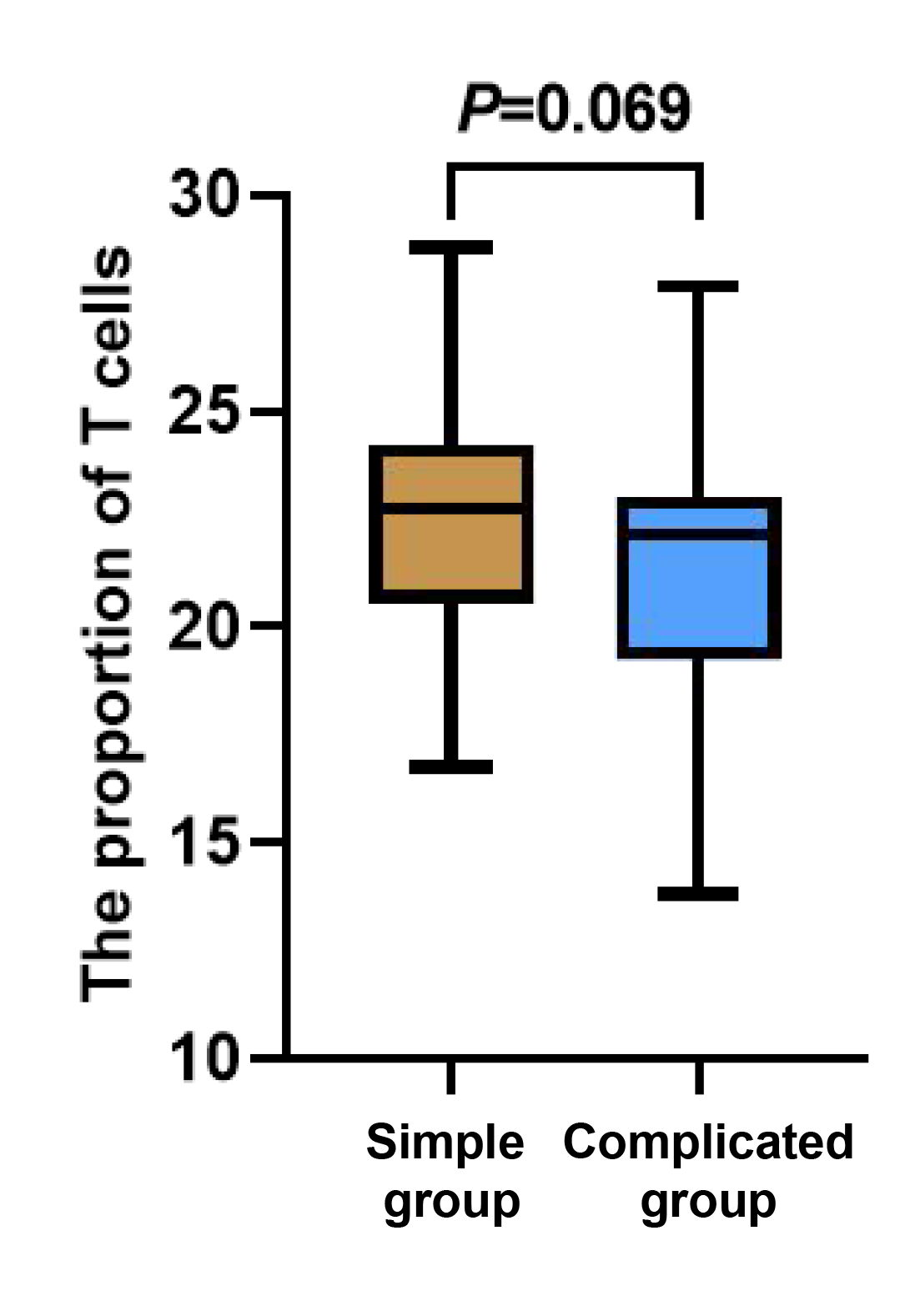
Figure 1 Between-group comparison of T cells.
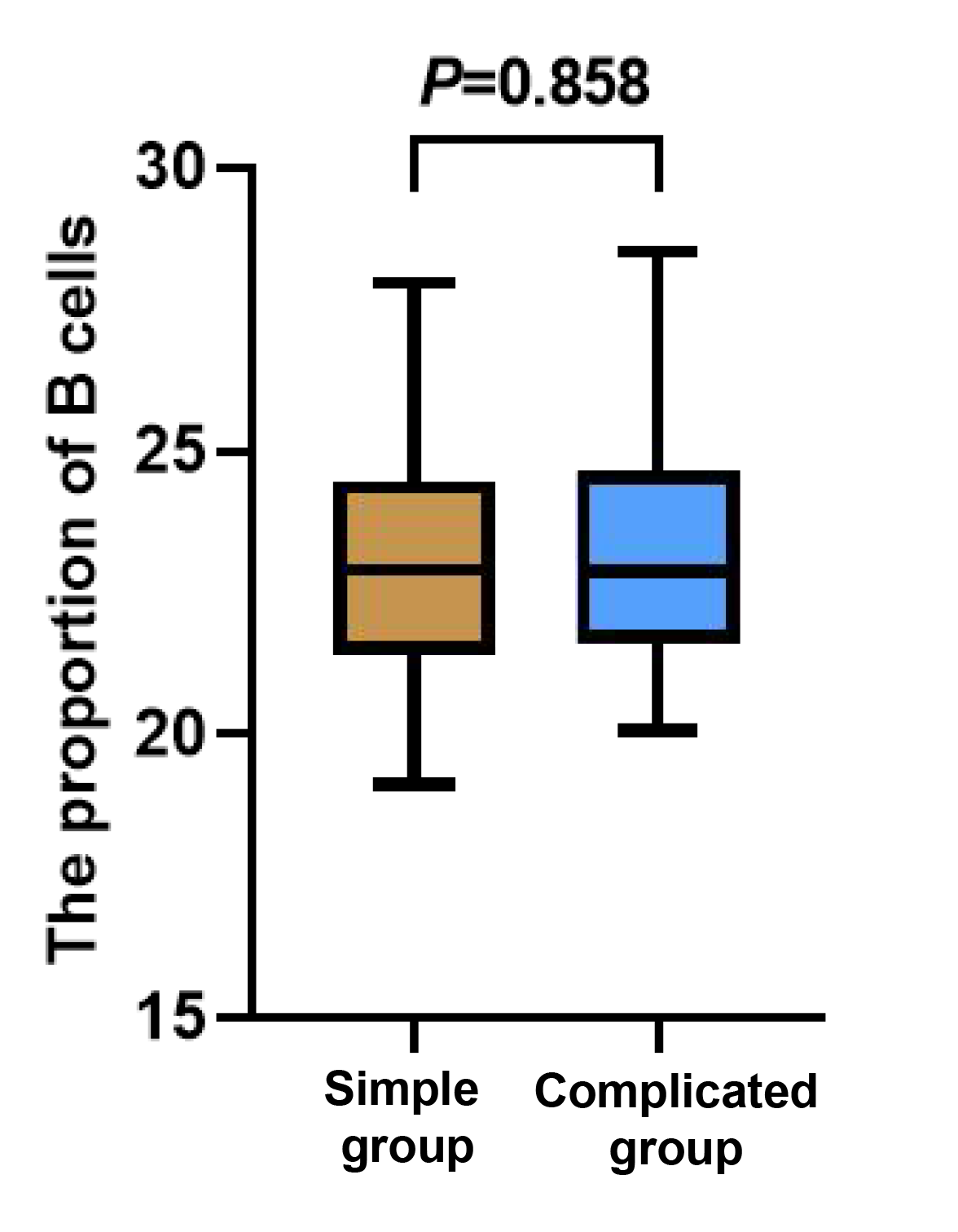
Figure 2 Between-group comparison of B cells.
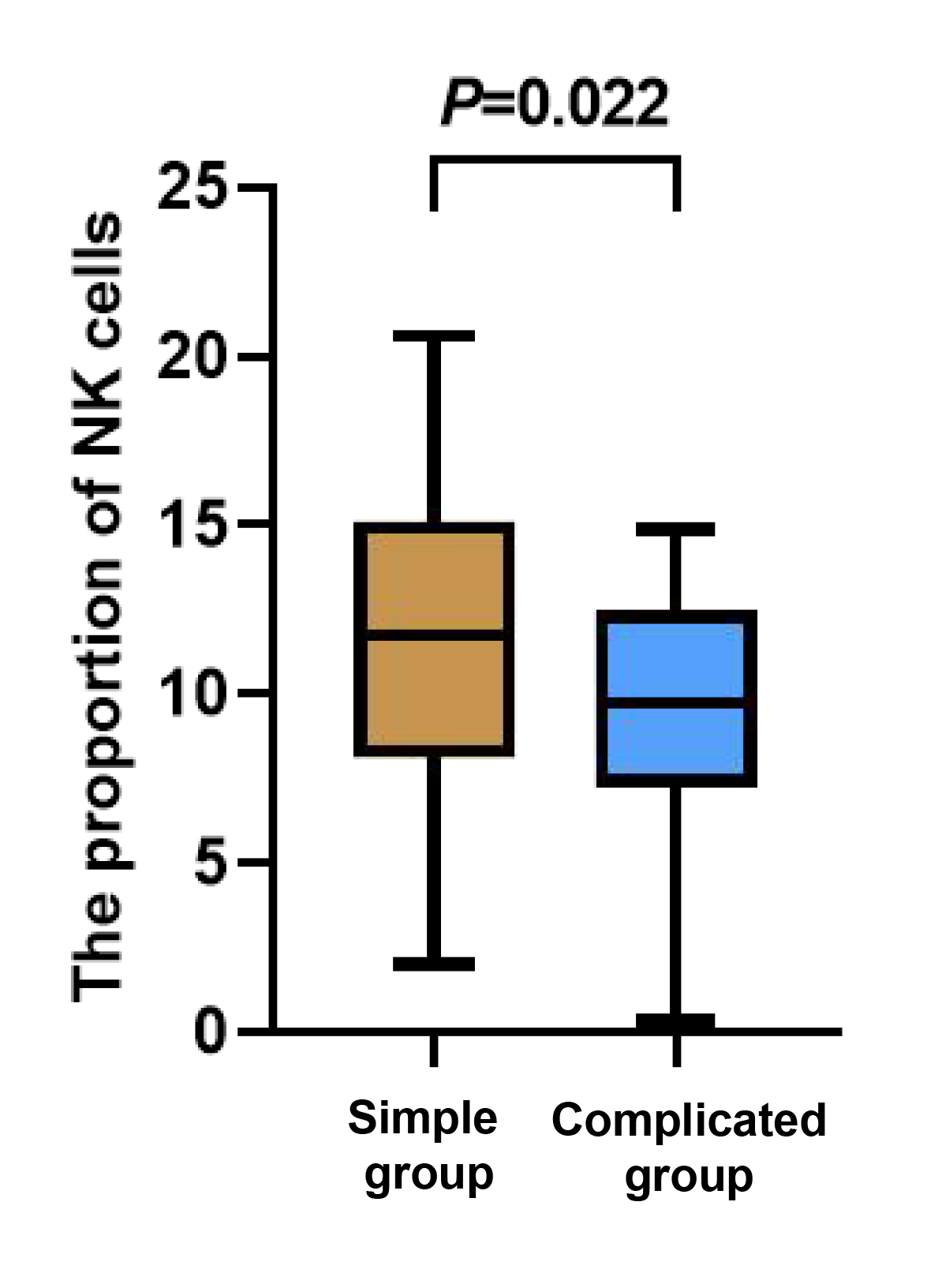
Figure 3 Between-group comparison of NK cells.
3.3 Immunoglobulin
The levels of IgA and IgG were evidently lower in the complicated group than those in the simple group (p < 0.05), while the level of IgE in the complicated group was apparently higher than that in the simple group (p < 0.05, Figures 4-6).
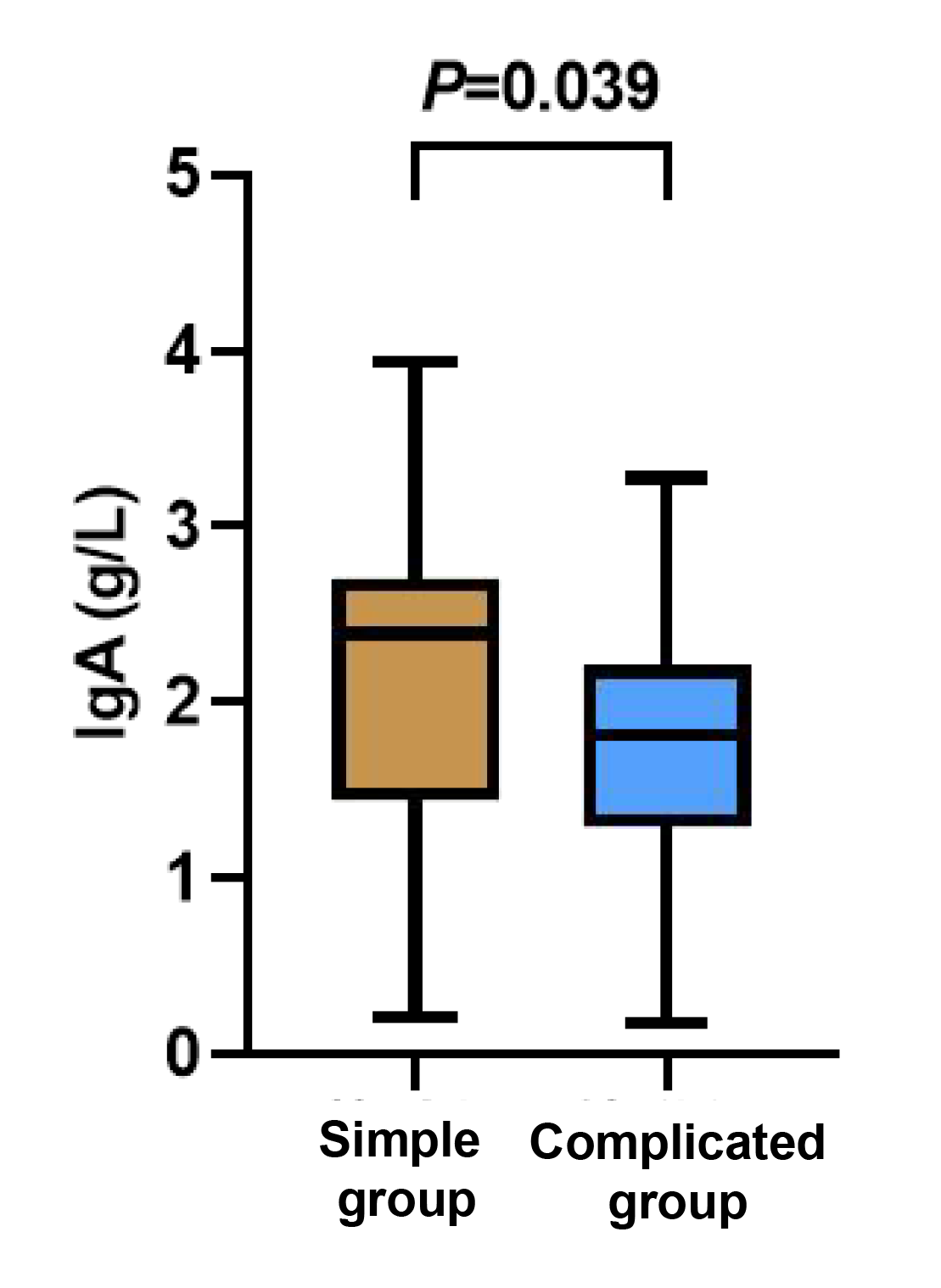
Figure 4 Between-group comparison of IgA cells.
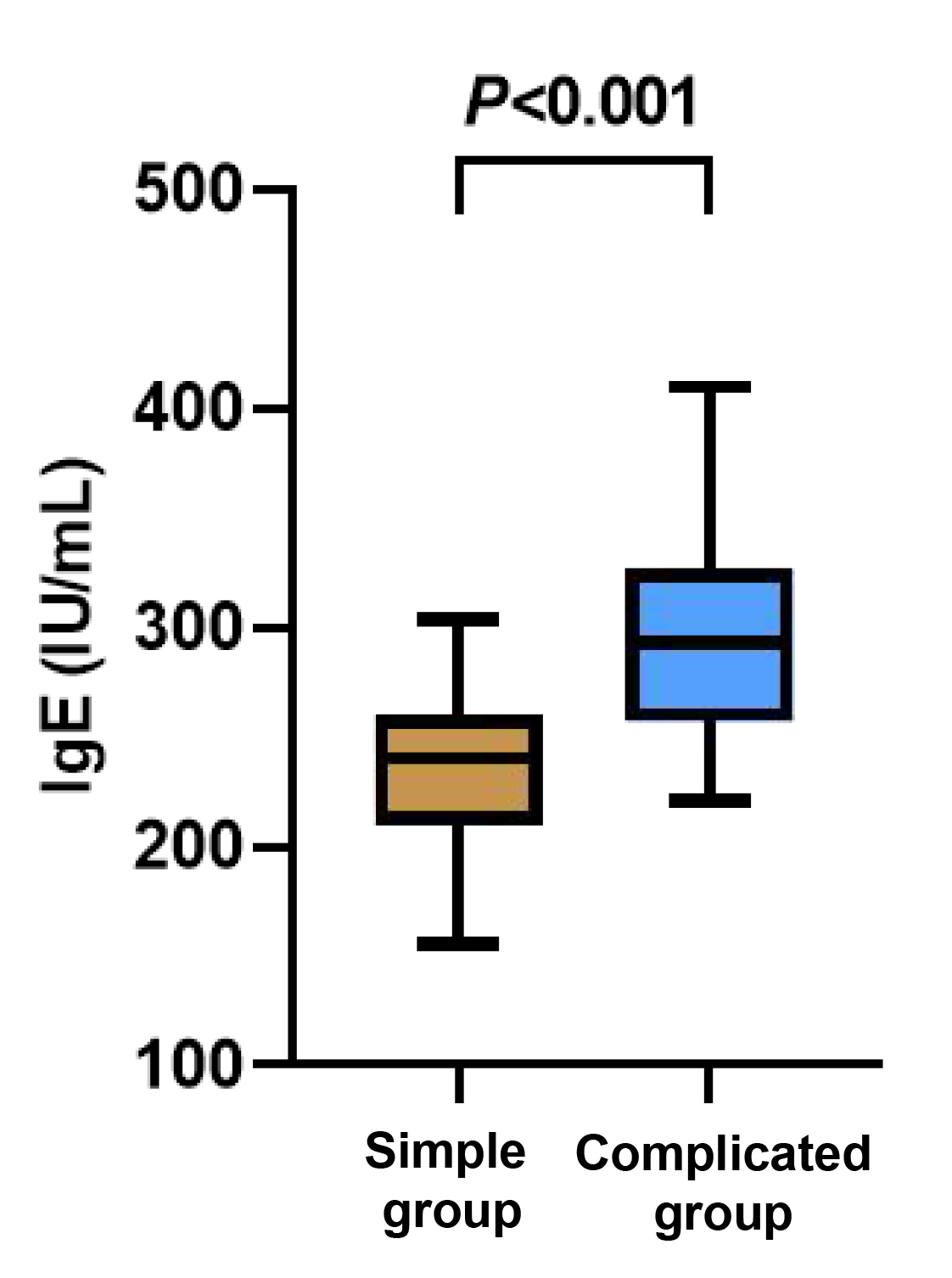
Figure 5 Between-group comparison of IgE cells.
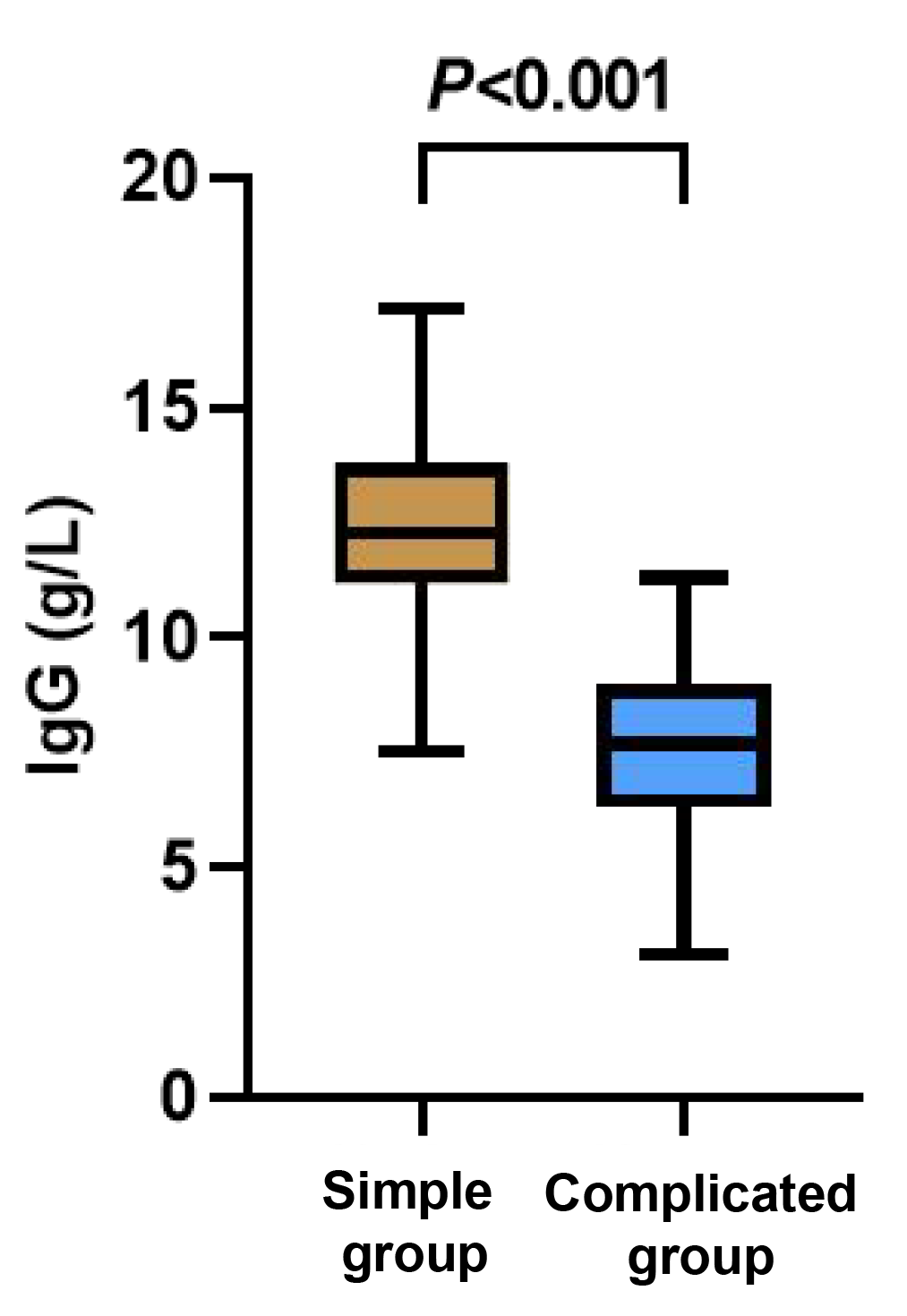
Figure 6 Between-group comparison of IgG cells.
3.4 Relevance
NK cells, IgA level and IgG level were negatively correlated (p < 0.05), while IgE levels were positively correlated with gastrointestinal symptoms in Henoch-Schonlein purpura (p < 0.05, Table 2).
Table 2 Relevance analyses of each indicator.
| Indicators | r | p |
|---|---|---|
| NK | -0.256 | 0.011 |
| lgA | -0.232 | 0.019 |
| lgE | 0.581 | < 0.001 |
| lgG | -0.794 | < 0.001 |
3.5 ROC analyses
The AUC values of NK cells, IgA level, IgE level and IgG level for diagnosing gastrointestinal symptoms in children with Henoch-Schonlein purpura were 0.649, 0.644, 0.829, and 0.969, respectively (p < 0.05, Table 3).
Table 3 ROC curve analysis of various indicators for diagnosing gastrointestinal symptoms in children with Henoch-Schonlein purpura.
| Indicators | AUC | Sensitivity degree | Specificity degree | The best critical point | p |
|---|---|---|---|---|---|
| NK | 0.649 | 0.975 | 0.325 | 14.600 | 0.021 |
| lgA | 0.644 | 0.725 | 0.650 | 2.010 | 0.027 |
| lgE | 0.829 | 0.675 | 0.900 | 272.485 | < 0.001 |
| lgG | 0.969 | 0.950 | 0.925 | 10.060 | < 0.001 |
4 Discussion
The diagnosis of gastrointestinal symptoms in children with Henoch-Schonlein purpura is of great significance for their treatment. To improve diagnostic accuracy, this study explored the diagnostic value of lymphocyte subsets combined with immunoglobulins for this disease. The results showed that lymphocyte subsets combined with immunoglobulins may have good diagnostic value for children with Henoch-Schonlein purpura complicated by gastrointestinal symptoms.
Lymphocyte subsets and immunoglobulins are the main indicators reflecting the cellular immunity of the body, among which NK cells are important immune cells. The lower NK cell count and levels of IgA and IgG and the higher IgE level indicated worse immune function of the body [13-15]. Herein, children with Henoch-Schonlein purpura complicated by gastrointestinal symptoms had lower NK cell counts and levels of IgA and IgG, but higher IgE levels compared to those with simple purpura. Moreover, NK cell counts and levels of IgA and IgG were negatively correlated with gastrointestinal symptoms in children with Henoch-Schonlein purpura, while IgE levels were positively correlated. This suggested that NK cells and the levels of IgA, IgE and IgG may play important roles in the diagnosis of Henoch-Schonlein purpura complicated by gastrointestinal symptoms. Reportedly [16], if a child with Henoch-Schonlein purpura develops concurrent infections, renal involvement, and systemic distribution of purpura, it indicates more severe actual condition, with more affected organs and a higher risk of gastrointestinal symptoms. Abnormal immune system and immune inflammation are the main potential pathogenic mechanisms that induce HSP. Children with Henoch-Schonlein purpura, under the stimulation of antigenic substances, can cause an imbalance of B cell and T cell subsets in the body. The immune dysfunction of T lymphocytes further induces the uncontrolled secretion of inflammatory mediators in B cells and tissues, stimulating their production of antibodies and resulting in immune dysfunction [17,18]. NK cells enhance the immune effects of T cells by activating macrophages within the body, inhibit the activation of B cells, or suppress the antigen-presenting function of helper cells, thereby regulating antibody production [19]. In areas rich in capillaries, such as the kidneys, a reduction in NK cells can trigger the accumulation of foreign antigens, inducing a strong immune response and causing renal damage. As more organs are involved and the disease progresses, gastrointestinal symptoms are more likely to occur [20]. IgA and IgG are the main components of the body's immune defense, while IgE is an antibody that mediates type I allergic reactions. Children with Henoch-Schonlein purpura have more severe immune dysfunction and are in an allergic state, presenting lower IgA and IgG levels compared to children with purpura simplex, and their immune function is weakened, making them more prone to co-infection symptoms. The worsening of the condition increases the risk of developing gastrointestinal symptoms [21-23]. Therefore, the counts of NK cells and the levels of IgA, IgG and IgE may have a close correlation with Henoch-Schonlein purpura complicated by gastrointestinal symptoms and have certain diagnostic value. Their level changes can be used to assist in the diagnosis of clinical symptoms and the formulation of corresponding treatments, consistent with Jie Huang et al.'s study [24].
According to the ROC curves, the AUC values of NK cells, IgA level, IgE level and IgG level for diagnosing gastrointestinal symptoms in children with Henoch-Schonlein purpura were 0.649, 0.644, 0.829, and 0.969, respectively, implying that NK, IgA, IgE, and IgG can all be used to diagnose gastrointestinal symptoms in children with Henoch-Schonlein purpura, with higher combined predictive value.
In conclusion, the combination of lymphocyte subsets and immunoglobulins may have good diagnostic values for children with Henoch-Schonlein purpura complicated by gastrointestinal symptoms. The decrease in NK cell count and IgA/IgG levels, as well as the increase in IgE levels, may indicate gastrointestinal symptoms in children with Henoch-Schonlein purpura.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: X.R.; Data curation: L.Z.; Formal analysis: G.C.; Methodology: L.Z.; Writing – original draft: X.R.; Writing – review and editing: X.R. and Z.Y.; All authors have read and agreed to the published version of manuscript.
Ethics Approval and Consent to Participate
This study was approved by the Medical Ethics Committee (Lun Approval No. 2401), and all the family members of the children patients signed the informed consent form.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Availability of Data and Materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Supplementary Materials
Not applicable.
References
- Du LN, Wang PP, Liu C, et al. Multisystemic manifestations of IgA vasculitis. Clinical Rheumatology 2021; 40(1): 43-52.
- Jia JK, Shen Q, Yu M, et al. Diagnosis and treatment analysis of children with abdominal Henoch-Schönlein purpura complicated with severe intussusception. Journal of Clinical Emergency 2016; 17(4): 320-321.
- Leung AKC, Barankin B, Leong KF. Henoch-Schönlein Purpura in children: an updated review. Current Pediatric Reviews 2020; 16(4): 265-276.
- Asiri A, Alzahrani F, Alshehri S, et al. New-onset Henoch-Schönlein Purpura after COVID-19 infection: a case report and review of the literature. Case Reports in Pediatrics 2022; 2022: 1712651.
- Li MJ, Feng AP, Liu XX, et al. Correlation analysis of food intolerance in patients with primary and recurrent abdominal Henoch-Schönlein purpura. Chinese Journal of Integrated Traditional and Western Medicine on Digestion 2016; 24(10): 771-775.
- Zhou YY, Wang Q, Shao DM, et al. Discussion on the correlation between Helicobacter pylori and various allergic diseases based on "spleen-stomach dysfunction". Tianjin Journal of Traditional Chinese Medicine 2023; 40(4): 447-451.
- Fang Y, Peng K, Zhao H, et al. The characteristics of video capsule endoscopy in pediatric Henoch-Schönlein purpura with gastrointestinal symptoms. Pediatric Rheumatology Online Journal 2020; 18(1): 84.
- Yang Y, Shu J, Mu J, et al. Clinical analysis of 99 children with Henoch-Schönlein purpura complicated with overt gastrointestinal bleeding. Clinical Rheumatology 2022; 41(12): 3783-3790.
- Zheng LJ, Yu J, Li XQ, et al. Analysis of clinical value of laboratory test indicators in children with Henoch-Schönlein purpura. Chinese Pediatrics of Integrated Traditional and Western Medicine 2019; 11(6): 499-502.
- Paek EY, Yi DY, Kang B, et al. Fecal calprotectin as a marker of gastrointestinal involvement in pediatric Henoch-Schönlein purpura patients: a retrospective analysis. BMC Pediatrics 2020; 20(1): 374.
- Cai LL, Hao WM, Xia HL. Application of lymphocyte subset detection in diagnosis and treatment of children with Henoch-Schönlein purpura. Anhui Medical and Pharmaceutical Journal 2018; 22(8): 1507-1510.
- Subspecialty Group of Immunology, Society of Pediatrics, Chinese Medical Association, et al. Evidence-based recommendations for diagnosis and treatment of childhood Henoch-Schönlein purpura. Chinese Journal of Pediatrics 2013; 51(7): 502-507.
- Wang LQ, Wu C, Liu XY, et al. Clinical efficacy of Tuizi Granules on recurrent rash in children with Henoch-Schönlein purpura and its effect on immune function. Modernization of Traditional Chinese Medicine and Materia Medica-World Science and Technology 2022; 24(10): 4125-4131.
- An R, Chen Y, Zhang SJ, et al. Significance of immune-inflammatory indicators and lymphocyte subsets in prognosis of newly diagnosed multiple myeloma. Journal of Clinical Hematology 2022; 35(3): 168-173.
- Bai JW. Changes of peripheral blood T lymphocyte subsets and significance of immune function detection in children with Henoch-Schönlein purpura. Jilin Medical Journal 2022; 43(6): 1646-1647.
- Li YL, Zhang J, Cao GH. Analysis of influencing factors of gastrointestinal involvement in Henoch-Schönlein purpura and nursing strategies. Journal of Community Medicine 2020; 18(20): 1416-1418.
- Wang B, Shao KFR, Dong C. Expression and significance of follicular helper T cells and galactose-deficient IgA1 in children with Henoch-Schönlein purpura. Chinese Journal of Contemporary Pediatrics 2020; 22(5): 473-477.
- Liu M, Wang J, Sun H, et al. Changes of immune function in children with Henoch-Schönlein purpura and its clinical significance. Laboratory Medicine and Clinic 2019; 16(3): 364-366.
- Becker PS, Suck G, Nowakowska P, et al. Selection and expansion of natural killer cells for NK cell-based immunotherapy. Cancer Immunology, Immunotherapy 2016; 65(4): 477-484.
- Xu J, Shi JB, Xie AM, et al. Serum CD4+/CD8+T and NK cell levels in children with Henoch-Schönlein purpura and their correlation with disease severity. Henan Medical Research 2020; 29(7): 1297-1299.
- Yu LJ, Tang YX, Yu ZC, et al. Expression of IL-17, IgA and IgE in children with Henoch-Schönlein purpura and analysis of risk factors affecting recurrence. Modern Preventive Medicine 2023; 50(1): 86-90.
- Kanagaratham CEl, Ansari YS, Lewis OL, et al. IgE and IgG antibodies as regulators of mast cell and basophil functions in food allergy. Frontiers in Immunology 2020; 11: 603050.
- Wu YG, He F. Logistic regression analysis of risk factors for renal damage in children with Henoch-Schönlein purpura. Chinese Journal of Dermatovenereology of Integrated Traditional and Western Medicine 2019; 18(5): 397-400.
- Huang J. Correlation between Henoch-Schönlein purpura and levels of immunoglobulins and T lymphocyte subsets. Dermatology and Venereology 2020; 42(1): 18-19.