Main Text
1 Introduction
Epimedium brevicornu Maxim., also known as Sanzhi Jiuye Cao, Xianlingpi, or Gangqian, has leaves as its medicinal part. It has a warm nature, a pungent and sweet taste, and the effects of tonifying kidney yang, strengthening muscles and bones, and dispelling wind and dampness [1]. According to modern pharmacological research, Epimedium brevicornu Maxim. has regulatory effects on the cardiovascular system, immune system, blood lipids, and blood glucose, and has various pharmacological effects such as improving immune function, anti-osteoporosis, antioxidant, anti-inflammation, anti-tumor, reducing blood glucose, and antidepression. Among them, flavonoids are the main active ingredients [2]. In most types of Epimedium brevicornu Maxim. preparations, the content of flavonoid compounds follows the order: Epimedin C > Epimedin B > Icariin > Baohuoside I > Epimedin A > Icaritin [3]. Research has shown that total flavonoids of Epimedium brevicornu Maxim. can be used for anti-osteoporosis, anti-myocardial hypoxia, anti-tumor, and immunoregulation [4].
Tunneling nanotubes (TNTs) are one of the communication methods between animal cells, which was first observed by RUSTOM et al. in 2004 in rat pheochromocytoma cells (PC12), human embryonic kidney cells (HEK), and normal rat kidney cells (NRK) [5]. TNTs are an extremely fine membrane nanochannel capable of transferring substances such as proteins and mitochondria. TNTs are different from previous gap junctions and are a novel form of intercellular communication. Gap junctions are mostly responsible for short-range communication between cells, while TNTs can achieve long-distance and directional communication between cells. Currently, TNTs have been found in various types of cells such as nerve cells, immune cells, and tumor cells [6-8]. TNTs can mediate the transmission of genetic information and long-distance transmission of Ca2+. For example, microRNA-155 can transfer between bladder cancer cells RT4 and T24 through TNTs, enhancing the invasion and proliferation of bladder cancer [9]. The TNTs formed between two independent pericytes in the capillary system are called interpericyte tunnelling nanotubes (IP-TNTs) and can form a functional TNTs network in the mouse retina [10]. In addition, due to their sufficiently large inner diameter, TNTs can facilitate the transfer of organelles such as mitochondria, Golgi apparatus, lysosomes, and intracellular vesicles between cells [11]. The main pathway for mitochondrial transfer between cells is the transport of TNTs [12]. Under stress conditions, TNTs can also act as a survival mechanism for cells. Melatonin can promote mitochondrial transfer between damaged HT22 cells via TNTs [13]. Mitochondrial transport via TNTs between certain cell types can also be bidirectional. The TNTs present between mesenchymal stem cells and umbilical vein endothelial cells can mediate the bidirectional transport of mitochondria between cells, facilitating cell osteogenic and angiogenic abilities [14,15]. In the field of bone tissue regeneration, the TNT-mediated intercellular mitochondrial transport can promote the process of bone regeneration [16].
Research reported that the main flavonoid components of Epimedium brevicornu Maxim., such as Epimedin C, Epimedin B, Icariin, and Baohuoside I, can act on bone metabolism [17]. TNTs can boost bone regeneration by mediating intercellular mitochondrial transport. It can be reasonably inferred that the main flavonoids in Epimedium brevicornu Maxim. may directly or indirectly enhance the formation of TNTs in the process of exerting biological effects. Therefore, this study selected Epimedin C, the most abundant flavonoid in Epimedium brevicornu Maxim., as a representative compound to preliminarily explore its role and mechanism in promoting TNTs production.
Against the backdrop of complex biological networks, network pharmacology integrates compound structures, biological effects, and relevant targets to construct molecular interaction networks. This approach has emerged as a crucial research strategy for elucidating the effective components, targets, and mechanisms of traditional Chinese medicine. In recent years, there has been accumulated research on the morphological characteristics, formation mechanisms, regulatory factors, and functions of TNTs in the development of various diseases [10]. As a potential active ingredient in the prevention and treatment of osteoporosis, a systematic clarification of its stimulatory effect on TNT production and the underlying molecular mechanism will not only contribute to the development of a new approach for osteoporosis treatment but also provide a theoretical foundation for disease treatment strategies centered on the regulation of intercellular junctions. Therefore, in this study, we employed network pharmacology to systematically forecast the potential targets of Epimedin C in regulating TNT formation, establish a protein interaction network, and preliminarily investigate the mechanism by which Epimedin C promotes TNT formation. Aiming to offer innovative theoretical support for the in - depth research and clinical application of this component.
2 Materials and methods
2.1 The obtainment of targets of Epimedin C
Using PubChem database (https://pubchem.ncbi.nlm.nih.gov/), the mol file and SMILES of the chemical structure of Epimedin C were retrieved. Then the obtained mol file was uploaded to Pharm Mapper database (http://lilab-ecust.cn/pharmmapper/index. Html) to predict the action targets of Epimedin C. With Norm Fit > 0.5 as the screening criterion, the obtained target names were standardized through the UniProt database (https://www.uniprot.org/). The SMILES structural formula was also imported into the Swiss Target Prediction database (http://swisstargetprediction.ch/), and targets of Epimedin C were predicted with a probability threshold of > 0. The results from both databases were integrated to acquire common targets.
2.2 Screening of TNTs targets and common target genes
With "tunneling nanotubes" as the keyword in GeneCards database (https://www. genecards. org/), the targets of TNTs were identified. The targets of Epimedin C and TNTs were uploaded to the bioinformatics online platform (https://www.bioinformatics.com.cn/) to generate a Venn diagram, and their intersection was taken to obtain potential targets of Epimedin C promoting TNTs generation.
2.3 Protein-protein interaction (PPI) network construction
To further understand the functions of the selected target protein genes and their roles in signaling pathways, the common targets were imported into the Metascape database [18]. By inputting a list of target gene names and limiting the species to humans, a threshold of p < 0.01 was set for Gene Ontology (GO) biological process enrichment analysis and Kyoto Encyclopedia of Genes and Genomes (KEGG) signaling pathway enrichment analysis. Finally, the obtained results were analyzed.
2.4 GO functional enrichment analysis and KEGG pathway enrichment analysis
The David database (https://david.ncifcrf.gov/) was used to perform GO and KEGG enrichment analysis on intersecting genes. The top 10 results (all results were included if fewer than 10 were available) from the KEGG enrichment analysis and the GO analysis in Biological Process (BP), Molecular Function (MF), and Cellular Component (CC) were uploaded to the bioinformatics platform for visualization processing, with FDR as the X-axis, Term as the Y-axis, Count as the point size, and p Value as the colorbar condition. Corresponding enrichment bubble plots were plotted.
3 Results
3.1 Common targets of Epimedin C and TNTs
After screening the data from the Swiss Target Prediction database and Pharm Mapper database, we identified 16 and 232 targets of Epimedin C, respectively. Following merging and deduplication, a total of 243 targets of Epimedin C were obtained. A search in the GeneCards database yielded 157 targets related to TNTs. The intersection of the targets of Epimedin C and TNTs resulted in 11 intersection targets (Figure 1), and the detailed content of the intersection targets was recorded in Table 1.
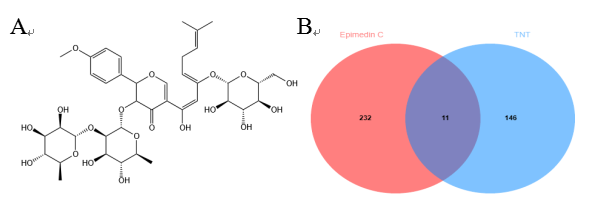
Figure 1 Prediction of common targets for Epimedin C in TNTs formation. (A) the chemical structure of Epimedin C. (B) Common targets of Epimedin C and TNTs.
Table 1 Common targets of Epimedin C and TNTs.
| Abbreviation of intersection target | Full name | Uniprot ID |
|---|---|---|
| ALB | Albumin | P02768 |
| IGF1 | Insulin Like Growth Factor 1 | P05019 |
| BMP2 | Bone Morphogenetic Protein 2 | P12643 |
| ACHE | Acetylcholinesterase (Cartwright Blood Group) | P22303 |
| MMP2 | Matrix Metallopeptidase 2 | P08253 |
| LCN2 | Lipocalin 2 | P80188 |
| EGFR | Epidermal Growth Factor Receptor | P00533 |
| CDK2 | Cyclin Dependent Kinase 2 | P24941 |
| SRC | SRC Proto-Oncogene, Non-Receptor Tyrosine Kinase | P12931 |
| ANXA5 | Annexin A5 | P08758 |
| BMP7 | Bone Morphogenetic Protein 7 | P18075 |
3.2 Construction of the PPI network for intersection targets of Epimedin C and TNTs
11 intersection targets were imported into a String database to construct a PPI network. The results showed that the network contained a total of 11 nodes, 33 edges, an average of 6 nodes, and an average local clustering coefficient of 0.841 (Figure 2, Table S1). According to the connectivity between the targets, each target was divided into three parts: red, blue, and green. The PPI network indicated that BMP2 and BMP7 were closely correlated, and EGFR, IGF1, MMP2, and SRC might interact with one another. ALB may be associated with LCN2, ACHE, ANXA5, and CDK2, and the subsequent study on the pathway mechanism offers some assistance.
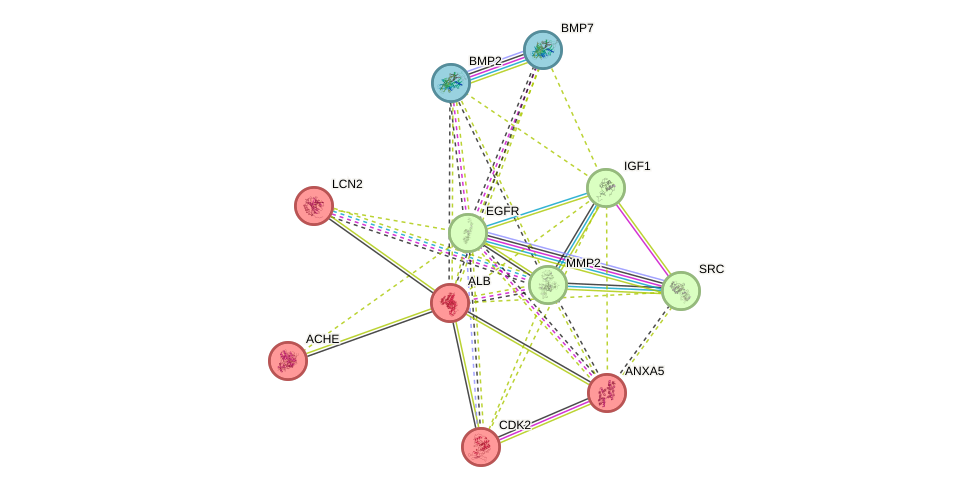
Figure 2 PPI network for intersection targets of Epimedin C and TNTs.
3.3 GO functional enrichment analysis and KEGG pathway enrichment analysis of intersection targets
11 intersection targets were uploaded to the David database for GO functional analysis and KEGG enrichment analysis. The results mainly revealed that the association between Epimedin C and TNTs involved 10 BP, 8 CC, and 10 MF in GO functional analysis. BP mainly involved the positive regulation of transcription, epithelial to mesenchymal transition, and the positive regulation of osteoblast differentiation (Figure 3, Table S2). CC primarily included components such as extracellular space, focal adhesion, and platelet alpha granule lumen (Figure 4, Table S3). MF was primarily associated with functions such as BMP receptor binding, binding energy, and protein binding (Figure 5, Table S4). KEGG pathway enrichment analysis revealed the action pathways of calycosin C on TNTs. The intersection targets mainly involve endocrine, bladder cancer, EGFR tyrosine kinase inhibitor resistance, and FoxO signaling pathway. (Figure 6, Table S5). These pathways and functions may play a key role in the process of Epimedin C promoting the generation of TNTs.
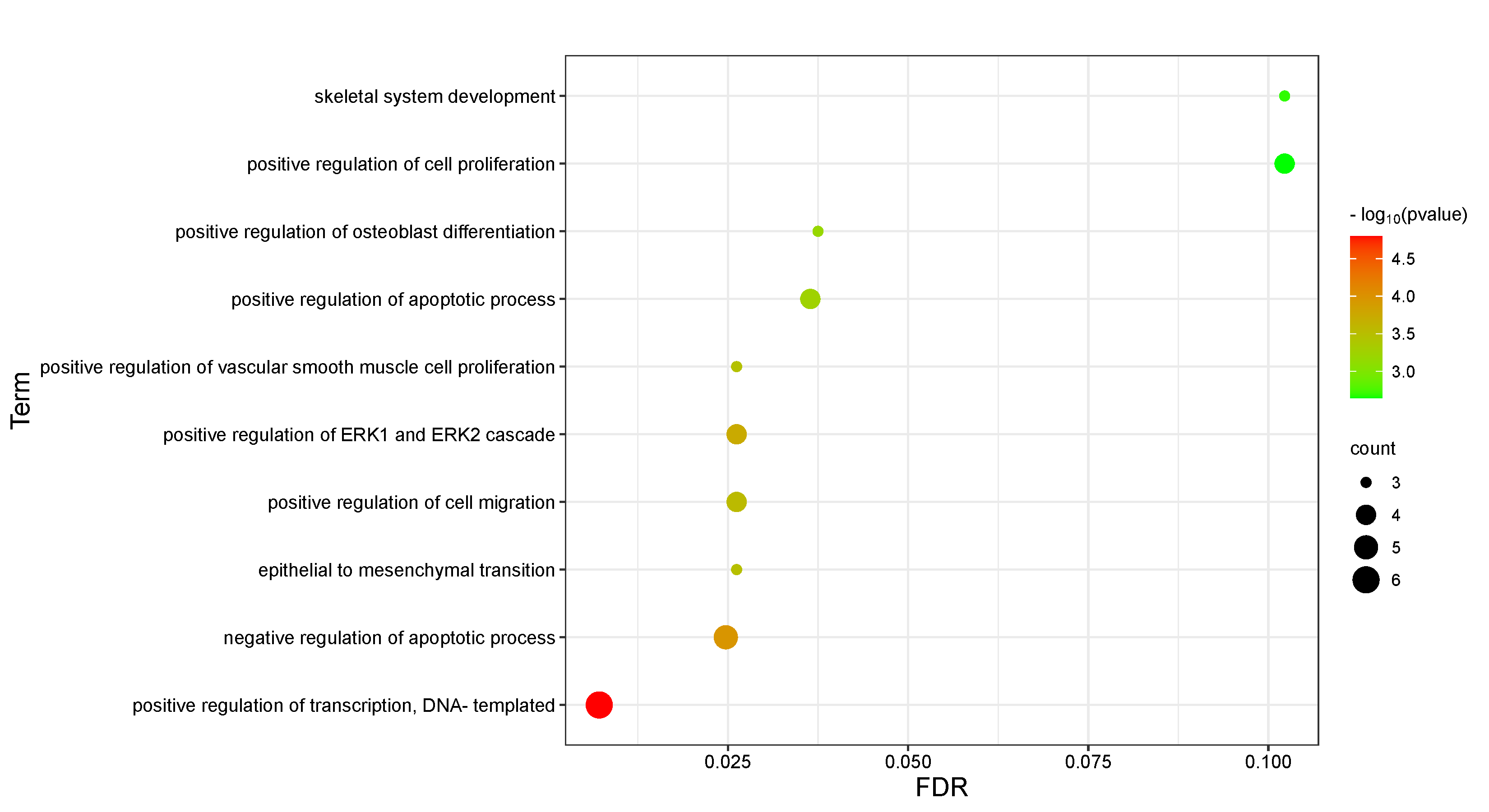
Figure 3 GO functional analysis of intersection targets (BP).
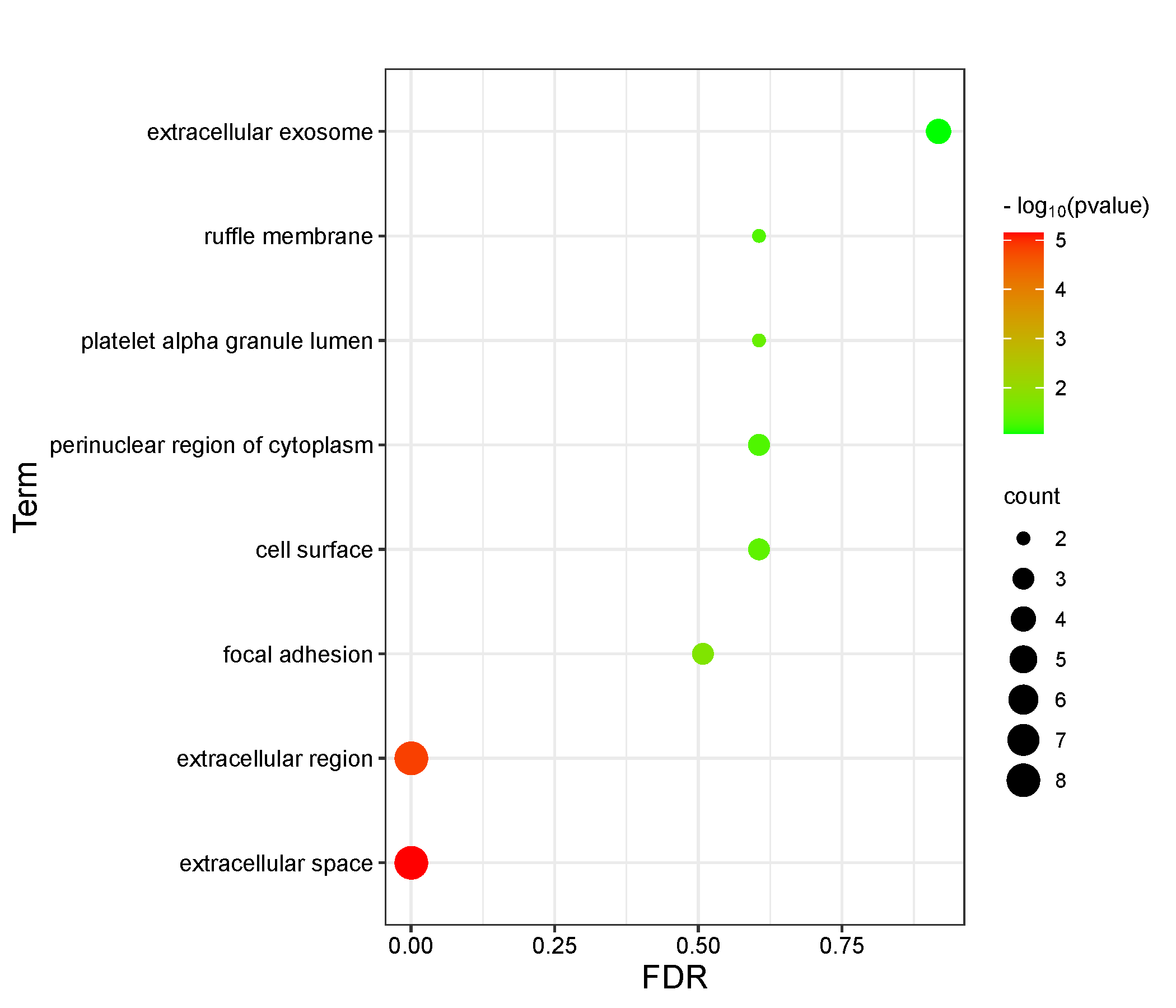
Figure 4 GO functional analysis of intersection targets (CC).
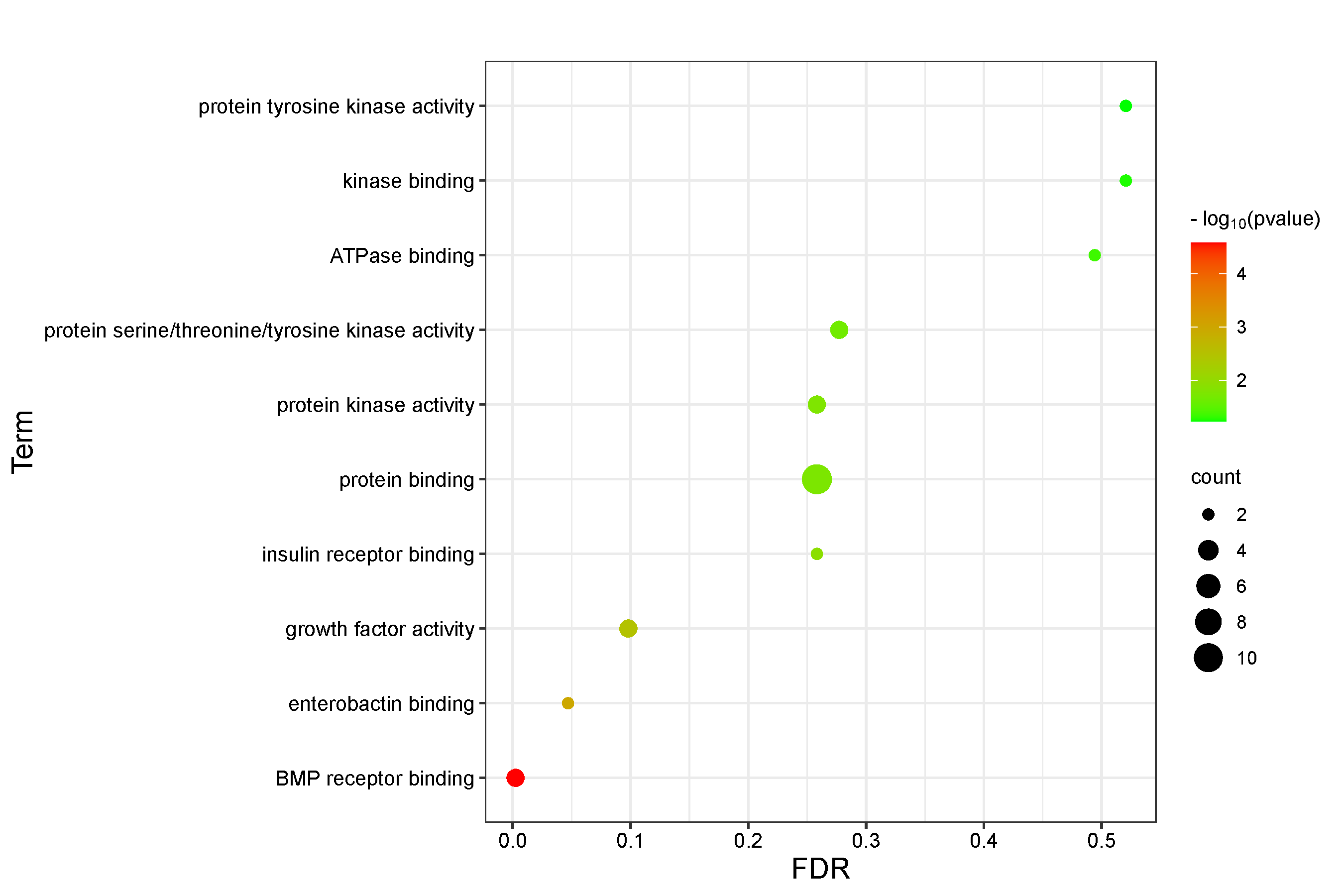
Figure 5 GO functional analysis of intersection targets (MF).
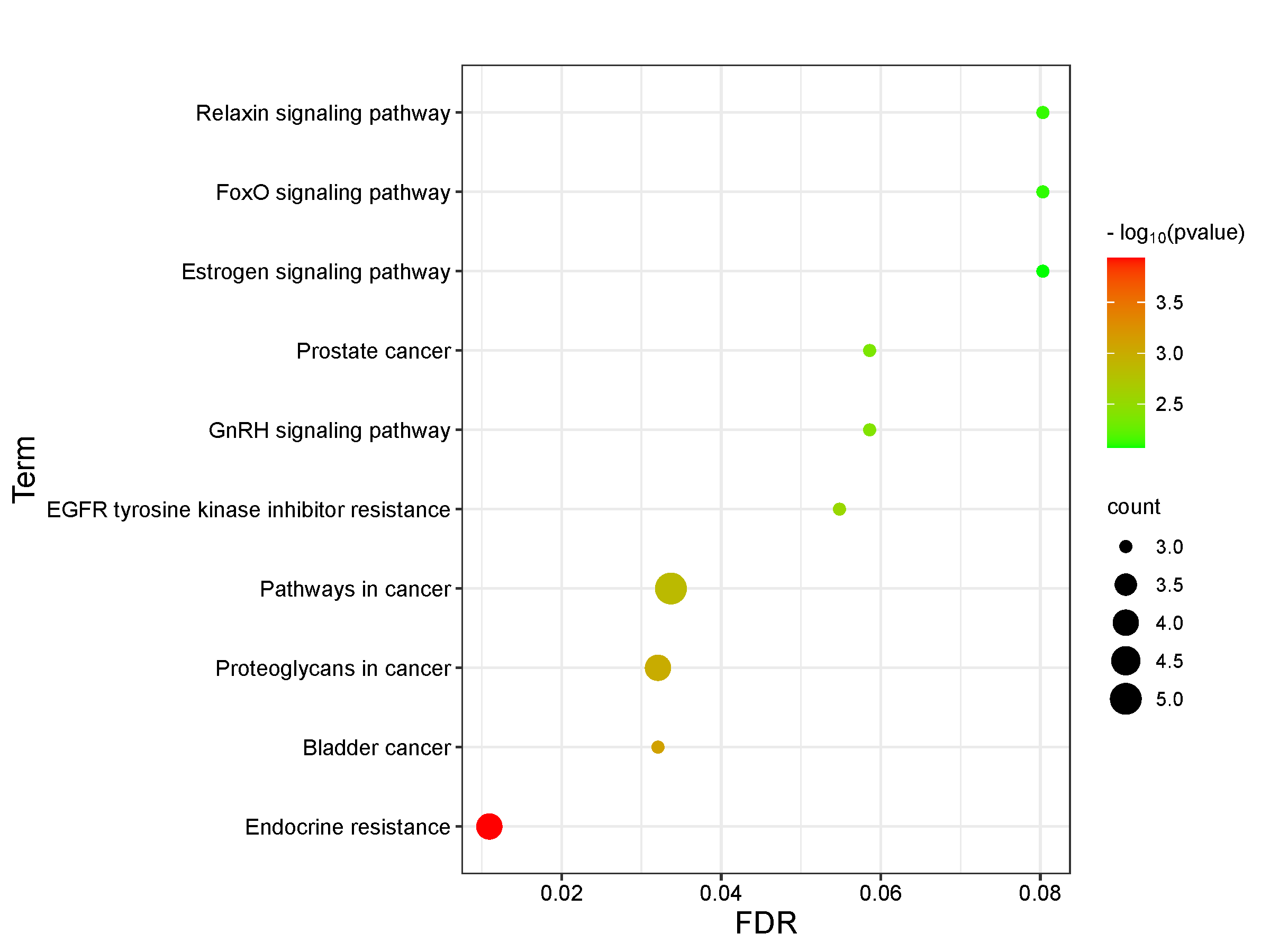
Figure 6 KEGG pathway enrichment analysis.
4 Discussion
This study preliminarily investigates the mechanism by which Epimedin C promotes the formation of TNTs using network pharmacology, providing a research direction for further exploration of corresponding mechanism. Total flavonoids are the main active ingredients of Epimedium brevicornu Maxim., in which Epimedin C accounts for the highest proportion. Modern research has shown that total flavonoids of Epimedium brevicornu Maxim. have beneficial effects on the immune system, endocrine tissues, bones and tumors, as well as the cardiovascular and cerebrovascular systems [16]. Total flavonoids of Epimedium brevicornu Maxim. can impact bone metabolism. The TNTs-mediated intercellular mitochondrial transport can promote bone regeneration. We can speculate that total flavonoids of Epimedium brevicornu Maxim. can promote the production of TNTs, but the relative mechanism remains obscure. Therefore, this study preliminarily explores the mechanism of Epimedin C boosting the generation of TNTs, with the aim of unveiling the role of Epimedin C in TNTs generation and providing a theoretical basis for subsequent research on Epimedin C-induced promotion of TNTs generation.
TNTs are mainly composed of intracellular skeletons and cell membranes such as F-actin and tubulin, which suspend above the substrates and connect different cells. TNTs contain single or multiple bundles of iTNT, each containing parallel actin filaments enveloped by a plasma membrane and connected by N-cadherin [10]. After treatment with actin inhibitors, the growth of TNTs in PC12 cells is significantly inhibited [5]. There are currently two known mechanisms for the formation of TNTs. One involves the extension of filopodia-like membrane protrusions, where multiple membrane protrusions protrude from the cell membrane. When one protrusion comes into contact with other cells, all but one protrusion retracts, leaving only one to form TNTs [19,20]. The other mechanism involves cell translocation, where two cells make direct contact, and membrane fusion occurs at the contact site. When the cells separate, the contact site is stretched and deformed, forming TNTs [21]. The duration of contact is the key to the formation of stable TNTs.
Through the PPI network diagram and KEGG enrichment analysis table of common targets, we can observe that the four targets in the green group and two targets in the blue group are closely related to Epimedin C and TNTs. These six targets are epidermal growth factor receptor (EGFR), insulin-like growth factor 1 (IGF1), matrix metalloproteinase 2 (MMP2), SRC oncogene (SRC), and bone morphogenetic protein 2/7 (BMP2/7). Upon combination with epidermal growth factor (EGF), EGFR can activate relevant genes in the nucleus, thereby promoting cell division and proliferation. During the process of cell proliferation, TNTs are also generated. IGF1 can bind to insulin-like growth factor 1 receptor (IGF-IR), which can regulate animal development and also plays an important role in muscle and bone growth, lymphocyte generation, and immunoregulation [22]. MMP2 is a critical member of the matrix metalloproteinase family, which is instrumental in angiogenesis along with extracellular matrix metalloproteinase inducer (EMMPRIN) [23]. SRC may participate in mediating embryonic development and cell growth [24]. BMP2/7 can affect mesenchymal stem cells and promote their osteogenic differentiation [25]. In summary, Epimedin C may promote the generation of TNTs by regulating targets such as EGFR, IGF1, MMP2, SRC, and BMP2/7.
GO analysis data show that the intersection targets mainly participate in biological processes such as transcriptional positive regulation, negative regulation of apoptosis, positive regulation of ERK1 and ERK2 cascade, cell proliferation and osteoblast differentiation, as well as epithelial to mesenchymal transition. KEGG analysis results reveal that the intersection targets are mainly enriched in signaling pathways such as endocrine, bladder cancer, resistance to EGFR tyrosine kinase inhibitors and FoxO signaling pathways. Epimedin C may may enhance EGFR expression in endocrine, prolonging the contact time between membrane protrusions and other cells [26,27] and thus boosting the generation of TNTs. The effect of EGFR tyrosine kinase inhibitor resistance on TNTs is also achieved by changing the duration of contact between the membrane protrusions and other cells. Bladder cancer mainly regulates the production of TNTs by influencing the remodeling process of F-actin [28,29]. The binding of CDK2 and IGF1 in the FoxO signaling pathway affects the remodeling ability of the plasma membrane, which then interacts with cytoskeletal effectors to facilitate the formation of membrane ruffling and protrusions [30], thereby promoting the generation of TNTs.
The regulatory factors that impact the generation of TNTs include epidermal growth factor receptor pathway 8 (Eps8), exocyte complex [31], Fyn/ROCK/p-paxillin signaling pathway [29], Wnt/Ca2+pathway [32], and myosin X (Myo10) [33]. According to the target analysis of each signaling pathway in Table S5, Epimedin C mainly enhances the generation of TNTs through two targets, EGFR and IGF1. Therefore, we surmise that endocrine and EGFR tyrosine kinase inhibitor resistance are the main signaling pathways through which Epimedin C promotes the generation of TNTs. Further experimental studies are required to determine which of these pathways has a more significant impact.
This study integrates network databases and computational prediction methods to obtain target information, screens intersection targets, and constructs a PPI network. Subsequently, GO functional enrichment analysis and KEGG pathway analysis are performed on target genes to preliminarily explore the mechanism of Epimedin C promoting TNTs generation. The research results can provide theoretical support for subsequent experimental studies on the promotion of TNTs production by Epimedin C, and also offer new insights into the application of Epimedin C in the treatment of TNTs-related diseases such as osteoporosis.
Although this study preliminarily predicted the potential targets and pathways of Epimedin C in regulating the generation of TNTs, there are still the following limitations: first, the network pharmacology analysis mainly relies on public databases and computational predictions, and the targets and pathways obtained have not been verified by in vitro and in vivo experiments, and their biological reality needs to be further confirmed; Second, the potential contribution of its metabolites was not considered for target prediction based on its prototype compound, which may undergo metabolic transformation in vivo. Thirdly, the database itself has update lag and species differences, and some target and pathway annotations may not be fully applicable to the mechanisms related to human osteoporosis. In the future, it is necessary to integrate experimental verification and in-depth mechanism research to more fully explain the regulatory effect of Epimedin C on TNTs.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
L.X.: writing-original draft, conceptualization. G.C.: methodology, validation, data curation. M.Z.: writing-review and editing. All data were generated in-house, and no paper mill was used. All authors agree to be accountable for all aspects of work ensuring integrity and accuracy.
Ethics Approval and Consent to Participate
No ethical approval was required for this article.
Funding
This research was supported by the National Natural Science Foundation of China (82274621).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
The following supporting information can be downloaded at: https://ojs.exploverpub.com/index .php/jecacm/article/view/326/sup. Supplementary Table S1: PPI network for intersection targets of Epimedin C and TNTs. Supplementary Table S2: GO functional analysis of intersection targets (BP). Supplementary Table S3: GO functional analysis of intersection targets (CC). Supplementary Table S4: GO functional analysis of intersection targets (MF). Supplementary Table S5: KEGG pathway enrichment analysis.
References
- Pharmacopoeia Commission of the People's Republic of China. Pharmacopoeia of the People's Republic of China; China Medical Science Press: Beijing, China 2020; p. 340.
- Ma H, He X, Yang Y, et al. The genus Epimedium: an ethnopharmacological and phytochemical review. Journal of Ethnopharmacology 2011; 134(3): 519-541.
- Jin XY, Jia XB, Sun E, et al. Research on variation regularity of five main flavonoids contents in Epimedium and processed Epimedium. China Journal of Chinese Materia Medica 2009; 34(21): 2738-2742.
- Li L, Wang JR, Wang J, et al. Research Progress on Main Chemical Components and Pharmacological Effects of Yinyanghuo (Epimedium brevicornu Maxim.) and Predictive Analysis on Its Quality Marker. Chinese Archives of Traditional Chinese Medicine 2023: 1-32.
- Rustom A, Saffrich R, Markovic I, et al. Nanotubular highways for intercellular organelle transport. Science 2004; 303(5660): 1007-1010.
- Nasoni MG, Carloni S, Canonico B, et al. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. Journal of Pineal Research 2021; 71(1): e12747.
- Jana A, Ladner K, Lou E, et al. Tunneling Nanotubes between Cells Migrating in ECM Mimicking Fibrous Environments. Cancers 2022; 14(8): 1989.
- Kato K, Nguyen KT, Decker CW, et al. Tunneling nanotube formation promotes survival against 5-fluorouracil in MCF-7 breast cancer cells. FEBS Open Bio 2022; 12(1): 203-210.
- Lu JJ, Yang WM, Li F, et al. Tunneling Nanotubes Mediated microRNA-155 Intercellular Transportation Promotes Bladder Cancer Cells' Invasive and Proliferative Capacity. International Journal of Nanomedicine 2019; 14: 9731-9743.
- Cao J, Deng Y, Gao L, et al. Research progress of tunnel nanotubes. The Journal of Practical Medicine 2023; 39(13): 1719-1723.
- Barutta F, Bellini S, Kimura S, et al. Protective effect of the tunneling nanotube-TNFAIP2/M-sec system on podocyte autophagy in diabetic nephropathy. Autophagy 2023; 19(2): 505-524.
- Huang QL, Shen L, Deng Y. Progress on Mitochondrial Transfer Mechanisms of Mesenchymal Stem Cell. Chinese Journal of Cell Biology 2019; 41(9): 1822-1831.
- Nasoni MG, Carloni S, Canonico B, et al. Melatonin reshapes the mitochondrial network and promotes intercellular mitochondrial transfer via tunneling nanotubes after ischemic-like injury in hippocampal HT22 cells. Journal of Pineal Research 2021; 71(1): e12747.
- He YY, Zhang H, Ji P. Effect of TNTs-mediated mitochondrial transfer between mesenchymal stem cells and endothelial cells on vascularization in vitro. Journal of Army Medical University 2022; 44(4): 310-319.
- He YY. Study on the effect of tunneling nanotubes on mitochondrial regulation of β-catenin during osteogenesis of mesenchymal stem cells/umbilical vein endothelial cell spheroids. Chongqing Medical University; 2022.
- He YY, Li LJ, Li YZ, et al. Application research of tunneling nanotubes in the field of bone tissue regeneration. Proceedings of the 10th National Oral Biomedicine Academic Conference and the 6th National Oral Outstanding Youth Forum: China, 2020. In Compilation of Papers of the Conference; Chinese Stomatological Association, Professional Committee of Oral Biomedicine: Beijing, China, 2020; p. 2.
- Song L, Wang LW, Zhou YT, et al. Study on the mechanism of Epimedin C against osteoporosis in vivo. Journal of Li-shizhen Traditional Chinese Medicine 2021; 32(3): 555-558.
- Gong HQ, Gao M, Chai YH, et al. Research progress on chemical constituents and pharmacological effects of Epimedium. Journal of Hubei Minzu University (Medical Edition) 2021; 38(4): 75-78.
- Lu J, Zheng X, Li F, et al. Tunneling nanotubes promote intercellular mitochondria transfer followed by increased invasiveness in bladder cancer cells. Oncotarget 2017; 8(9): 15539-15552.
- Wang F, Zhang Y, Zhu L. Tunneling Nanotube: a Novel Type of Signal Transmission in The Nervous System. Progress in Biochemistry and Biophysics 2021; 48(1): 54-63.
- Victoria GS, Arkhipenko A, Zhu S, et al. Astrocyte-to-neuron intercellular prion transfer is mediated by cell-cell contact. Scientific Reports 2016; 6: 20762.
- Mei XL, Li CY, Kuang LD, et al. Research progress on the relationship between insulin-like growth factor 1 receptor polymorphisms and production performance. Jiangsu Agricultural Sciences 2019; 47(2): 29-32.
- Zhou T, Li J. The role of matrix metalloproteinase-2 and its inducer in tumor development. Modern Medical Journal 2011; 39(2): 254-256.
- Guo-Ping Du, Wei Zhang. The Relationship between Tyrosine Kinase Oncogenes and Embryonic Development and Implantation. Foreign Medical Sciences (Family Planning Section) 2005; (3): 119-122.
- Yang SL, Zhang GQ, Li C, et al. Mechanism of microRNA-133a regulating the RUNX2/BMP2 signaling pathway in steroid-induced osteonecrosis of the femoral head. Journal of China Medical University 2023: 1-8.
- Pan PY, Zhao QY, Wang Y, et al. Observation of Intercellular Tunnel Nanotubes by High Content Analysis System Combined With Laser Scanning Confocal Microscope. Progress in Biochemistry and Biophysics 2023; 50(7): 1742-1754.
- Li AQ, Deng LH, Wang X. Effect of the components of tunneling nanotube on its formation and stability. Journal of Biomedical Engineering Research 2022; 41(2): 114-121.
- Yu Q, Chen DJ, Lou G. The advances of tunneling nanotubes in tumors. Journal of Modern Oncology 2022; 30(1): 139-143.
- Lu JJ, Hai JB, Li F, et al. Glucose promotes the formation of tunneling nanotubes between bladder cancer cells by up-regulating Fyn/Rho-associated kinase signaling pathway. Chinese Journal of Experimental Surgery 2018; 35(9): 1607-1609.
- Kast DJ, Dominguez R. IRSp53 coordinates AMPK and 14-3-3 signaling to regulate filopodia dynamics and directed cell migration. Molecular Biology of the Cell 2019; 30(11): 1285-1297.
- Zhang FF, Zhao YW, Wang T, et al. Advances in the study of cytoskeleton system regulating interactions between secretory vesicles and plasma membrane. Scientia Sinica (Vitae) 2022; 52(1): 107-120.
- Vargas JY, Loria F, Wu YJ, et al. The Wnt/Ca2+ Pathway is involved in interneuronal communication mediated by tunneling nanotubes. The EMBO Journal 2019; 38(23): e101230.
- Sun YY, Yang YF, Keller KE. Myosin-X Silencing in the Trabecular Meshwork Suggests a Role for Tunneling Nanotubes in Outflow Regulation. Investigative Ophthalmology & Visual Science 2019; 60(2): 843-851.