Main Text
1 Introduction
Running a marathon or half-marathon has become an increasingly popular personal challenge for many non-competitive athletes. Annually, approximately 414,000 people across North America, 349,000 people across Europe, and 800,000 people across China take part in a marathon [1]. Despite great concerns in the safety of amateur runners in marathons, the lack of physiological data restricts detailed and comprehensive assessment [2]. The growing enthusiasm for long-term endurance training or competition underpins the high cardiovascular risks [2,3]. In general, increases in heart rate and stroke volume are primarily responsible for cardiac output augmentation during acute exercise in competitive athletes, resulting in cardiac chamber enlargement and increased wall thickness and mass, collectively termed athlete's heart [4,5]. Relevant exercise physiology for competitive athletes can be subdivided into isometric (strength) and isotonic (endurance) exercises. Isometric exercises can lead to thickening of left ventricular (LV) walls without chamber dilation, while isotonic exercises stimulate dilation of cardiac chambers with mild LV wall thickness [6]. Myocardial fibers are arranged helically, generating a clockwise rotation of the LV base and a simultaneous counterclockwise rotation of the LV apex during systole when viewed apically, which produces an overall twisting motion of the heart. The LV twist supports the production of stroke volume during systole, and rapid untwisting drives ventricular filling during diastole [7].
Speckle tracking echocardiography (STE) can noninvasively quantify dynamic LV parameters (including strain, rotation, torsion, and twist), and detect subtle alterations in systolic function when the LV ejection fraction (LVEF) remains normal [8]. Recent STE studies have verified systolic and diastolic dysfunctions in recreational marathon runners [9,10]. Changes in LV dia stolic function following prolonged strenuous exercise appear to have a direct impact on both LA diastolic and systolic peak deformation [11]. However, limited research focuses on the athlete's heart associated with LV twist function that is largely influenced by the volume and wall thickness of ventricle in amateur marathon runners.
Clinically, understanding cardiac structural and functional adaptations in amateur marathon runners is essential. LV twist has shown promise as a measure to quantify dysfunction, beyond traditional markers of function, and LV twist mechanics may be a useful tool to distinguish between physiology and pathology in endurance runners. However, the temporal progression of LV twist among amateur marathon runners remains incompletely understood. In addition, Traditional Chinese Medicine (TCM) theory posits that individuals can be classified into distinct constitutional types (TCM body constitutions), which represent inherent predispositions in physiological function and resilience to stress. The Pinghezhi, or balanced constitution, is considered the ideal state of health and homeostasis.
On this basis, the present study used STE to evaluate the LV twist functions of amateur marathon runners. The assessments were conducted pre-marathon, immediately post-marathon, and during two recovery phases (1 hour and day 4 post-marathon).
2 Materials and methods
2.1 Study population
This prospective study was conducted by the Marathon Sports Medicine Research Institute, the Affiliated Hospital of Hangzhou Normal University, between January 2021 and September 2023. 123 amateur marathon runners from the Zhejiang University Running Group were enrolled. They engaged in regular training (more than three times a week, with a distance of more than 10 km each time), and had completed a formal full marathon (42.195 km) at least once, including one within preceding six months. All runners were questioned about medical history, and those with systemic hypertension, diabetes mellitus, renal disease, chronic obstructive airway disease, any pre-existing cardiac illness (regional or global wall abnormalities, atrial fibrillation, valvular heart disease, cardiomyopathy, and so forth), or who were aged < 20 or > 60 years were excluded. The runners were grouped into 23 short-term(ST) runners (≤ 6 months), 32 more extended-term(MET) runners (6 months–3 years), and 30 long-term (LT) runners (> 3 years) based on their duration of marathon participation. Echocardiographic studies were performed at three separate time points: (1) within three days prior to the marathon, (2) 1 hour after the marathon, and (3) 4 days after the marathon. Comparisons were made with 30 healthy controls recruited via public notice boards who had no regular exercise habits and did not participate in recreational or competitive sports. All subjects received information about this study and signed written informed consent, and the institutional ethics committee approved the study. This study was conducted in accordance with the principles outlined in the Declaration of Helsinki.
2.2 Standard echocardiography
Echocardiographic studies were performed using a standard echocardiography system (Vivid E95, GE, Horten, Norway) and a 3.5-MHz transducer with ECG monitoring. The left atrial and the LV dimensions were measured according to current recommendations established by the American Society of Echocardiography and European Association of Cardiovascular Imaging (EACVI) [12]. The LVEF was calculated using the modified biplane Simpson′s formula based on end-diastolic volume (EDV), end-systolic volume (ESV) and stroke volume (SV). Interventricular septum diastolic (IVSd), LV end-diastolic diameters (LVEDD), posterior wall thickness (PW), and left atrial anteroposterior diameters (LAD) were measured from the parasternal long axis using 2D imaging. Relative wall thickness (RWT) was determined as the ratio of the sum of IVSd and PW to the LVEDD. LV mass (LVM) was calculated using the formula described by Lang et al [13]. Early-diastolic velocity (E), late-diastolic atrial filling velocity (A) and the E/A ratio were measured from the mitral inflow profile in the apical four-chamber view. Doppler tissue imaging (DTI) analysis was performed by placing the sample volume at the septal and lateral mitral annulus to obtain early-diastolic velocities and late-diastolic velocities as well as the average values (Ea′). The E/E′ ratio was also estimated. All parameters were averaged over five cardiac cycles.
2.3 Speckle tracking echocardiography
After a standard clinical echocardiographic study, the apical long-axis, four- and two-chamber views, and the basal, middle, and apical short-axis planes were scanned using a high frame rate (at least 50 frames/sec) in standard grayscale B-mode imaging for speckle tracking. All images were obtained during breath-hold and were stored digitally in a cine loop format from 5 consecutive beats. The software automatically tracked the myocardial motion during the cardiac cycle. Speckle tracking echocardiography was performed using the EchoPAC multiparameter analysis workstation (version 204, Vingmed Ultrasound AS). All longitudinal, circumferential, rotational, and twist parameters were obtained according to previous studies [14,15]. The global longitudinal strain (GLS) was obtained from the strain values in the apical long-axis view and the two- chamber and three- chamber views. The global circumferential strain (GCS) was derived from the parasternal short-axis view at the basal, mid, and apical levels. Clockwise rotation was conventionally marked as negative value and counterclockwise rotation as a positive value when viewed from the LV apex to base. The degree of LV twist was defined as the net difference between the apical and basal LV rotation. Torsion refers to the LV twist normalized by diastolic longitudinal length of the long axis of the LV. The untwisting rate (UTR) was defined as (LV twist-LV rotation mitral valve opening)/(time difference between these two events). Changes in GLS (Δ GLS) before marathon and 1 hour after marathon were calculated, according to the following formula: Δ GLS = (GLS before marathon-GLS 1 hour after marathon)/GLS before marathon × 100 (%). Similarly changes in the other speckle tracking echocardiographic parameters were obtained: (before marathon - 1 hour after marathon) divided by the before marathon × 100 (%). All echocardiographic measurements were performed independently by 2 observers who were blinded to the clinical characteristics of the study population. Intra-observer and inter-observer variabilities of speckle tracking echocardiographic data were evaluated blindly in 10 amateur marathon runners and 10 controls by two sonographers.
2.4 Investigation on Traditional Chinese Medicine (TCM) constitution types of amateur marathon athletes
The main constitution types are divided into peace, qi and blood deficiency, yin deficiency, yang deficiency, phlegm-dampness, damp-heat, blood stasis, qi depression, and special constitution. The specific classification method is based on the TCM constitution scale and the "Diagnostic criteria for 9 constitutional types of Traditional Chinese Medicine".
2.5 Statistical methods
Statistical analyses were performed with the Statistical Package for Social Science Software (ver. 22) and GraphPad Prism (ver.8.1.1). To test the normality of the data, Shapiro-Wilk test was used. Normally distributed variables are expressed as mean ± standard deviation, while non-normally distributed variables as median with inter-quartile range (IQR), or numbers (%). Values between four or three groups using one-way analysis of variance (ANOVA) for normally distributed data, or Kruskal–Wallis test for non-normally distributed data. And two groups were compared using either t test or Mann–Whitney test for normally and non-normally distributed variables, respectively. Either chi-square tests or Fisher′s exact tests were applied to determine the dissimilarities for categorical variables. Linear regression analysis was performed to determine the correlation between parameters. All statistical analyses were 2-tailed, and a p < 0.05-value < 0.05 was indicated as statistical significance.
3 Results
3.1 Study population and baseline characteristics
A total of 123 amateur marathon runners were enrolled initially. 13 subjects were excluded due to missed examinations in the follow-up conventional echocardiographic investigation, 16 subjects due to failure to complete the marathon race, and 9 subjects due to insufficient image quality for analyses. The baseline clinical characteristics of the 85 amateur marathon runners and 31 controls are presented in Table 1. The average age of subjects is 29.34 ± 5.80 in ST runners, 39.60 ± 7.03 in MET runners, 41.59 ± 8.82 in LT runners and 39.35 ± 8.80 in control group. These groups were similar with respect to gender, height, body weight, body mass index (BMI), body surface area (BSA) and systolic blood pressures (SBP) and diastolic blood pressures (DBP) (Table 1, p > 0.05). Heart rate was significantly lower in the amateur marathon runner compared with the controls (Table 1, p < 0.001). Additionally, the MET and LT runner groups had significantly lower heart rates than the ST groups (Table 1, p < 0.05).
Table 1 Baseline characteristics of the study population.
| Variable | Amateur marathon runners | Controls (n = 31) | p | ||
|---|---|---|---|---|---|
| ST (n = 23) | MET (n = 32) | LT (n = 30) | |||
| Age (years) | 29.34 ± 5.80 * | 39.60 ± 7.03 ※ | 41.59 ± 8.82 ※ | 39.35 ± 8.80 | < 0.001 |
| Male n (%) | 17 (33) | 21 (66) | 21 (70) | 20 (65) | 0.129 |
| Height (cm) | 172.0 (168.0, 175.0) | 170.0 (167.5, 175.0) | 172.0 (168.0, 175.0) | 172.0 (168.0, 175.0) | 0.755 |
| Body weight (kg) | 65.73 ± 6.57 | 64.37 ± 6.52 | 67.31 ± 7.44 | 64.60 ± 6.71 | 0.257 |
| BMI (kg/m2) | 22.56 ± 1.36 | 22.08 ± 1.42 | 23.03 ± 1.67 | 22.30 ± 1.50 | 0.097 |
| BSA (m2) | 1.82 ± 0.12 | 1.80 ± 0.11 | 1.87 ± 0.12 | 1.83 ± 0.11 | 0.339 |
| Heart rate (bpm) | 62.74 ± 6.77 * | 56.23 ± 6.80 *,※ | 55.52 ± 6.16 *,※ | 76.00 ± 10.23 | < 0.001 |
| SBP (mm Hg) | 121.09 ± 5.96 | 121.07 ± 5.81 | 122.76 ± 7.02 | 121.39 ± 6.71 | 0.723 |
| DBP (mm Hg) | 69.52 ± 5.11 | 72.80 ± 8.53 | 75.28 ± 6.49 | 73.35 ± 7.82 | 0.056 |
Note: Normally distributed data are presented as mean ± standard deviation. Non-normally distributed data were expressed as median (IQR). ST, short time; MET, more extended-term; LT, long-term; BMI, body mass index; BSA, body surface area; SBP, systolic blood pressure; DBP, diastolic blood pressure. * p < 0.05 compared with controls. ※ p < 0.05 compared with ST.
3.2 Differential cardiac remodeling by training volume
Table 2 indicates standard echocardiographic parameters. LVEF was similar across all 4 groups and remained within normal ranges (Table 2, p = 0.387). IVSd, LVEDD, PW, RWT, EDV, ESV, SV and LVM were significantly higher in the LT runners group compared with the ST runners, the MET runners, and the controls (Table 2, p < 0.01). Similarly, the corresponding indexed parameters (LVEDDi, EDVi, ESVi, SVi, and LVMi) were elevated in the LT runners group (Table 2, p < 0.01). In contrast, the ST runners group showed no significant differences from the control group in these parameters (Table 2, p > 0.05). The MET runners group exhibited significantly higher values of IVSd, LVEDD, LVEDDi, PW, RWT, EDV, EDVi, ESV, and ESVi relative to the control group (Table 2, p < 0.05) and had significantly higher IVSd, PW, RWT, EDV, EDVi, ESV, and ESVi than ST runners group (Table 2, p < 0.05). LVM in MET runners group did not significantly differ from that in ST runners group (87.9 (73.1, 99.7) vs. 77.5 (65.9, 95.7)) (Table 2, p > 0.05). Regarding standard Doppler and DTI parameters, the LT runners group demonstrated significantly lower values of E, E/A ratio, E′a, and E/E′a ratio, and a significantly higher A value compared to ST runners, MET runners, and controls (Table 2, p < 0.01), which had no significant differences were observed among the ST, MET, and control groups for these parameters (Table 2, p > 0.05).
Table 2 Standard echocardiography characteristics of the study population.
| Variable | Amateur marathon runners | Controls (n = 31) | p | ||
|---|---|---|---|---|---|
| ST (n = 23) | MET (n = 32) | LT (n = 30) | |||
| IVSd (cm) | 8.90 ± 1.39 | 10.03 ± 1.36 **,※※ | 11.34 ± 1.69 **,※※,†† | 8.72 ± 1.17 | < 0.001 |
| LVEDD (cm) | 48.6 (45.6, 51.4) | 50.0 (47.0, 52.2) ** | 55.0 (54.0, 56.0) **,※※,†† | 47.0 (45.0, 49.0) | < 0.001 |
| LVEDDi (cm/m2) | 26.76 ± 1.64 | 27.49 ± 2.33 ** | 29.23 ± 1.67 **,※※,† | 26.07 ± 2.81 | < 0.001 |
| PW (cm) | 8.36 ± 0.89 | 8.84 ± 1.28 **,※※ | 9.86 ± 1.35 **,※※,†† | 8.02 ± 0.94 | < 0.001 |
| EDV (mL) | 103 (93.9, 116.0) | 112.0 (94.9, 126.5) *,※ | 136.0 (127.0, 140.0) **,※※,†† | 99.8 (89.7, 113.0) | < 0.001 |
| EDVi (mL/m2) | 58.45 ± 9.29 | 62.10 ± 10.80 *,※ | 71.09 ± 10.74 **,※※,†† | 57.83 ± 7.49 | < 0.001 |
| ESV (mL) | 38.3 (33.2, 45.00) | 42.4 (34.1, 48.3) *,※ | 52.6 (47.8, 55.3) **,※※,†† | 38.30 (32.6, 43.6) | < 0.001 |
| ESVi (mL/m2) | 21.52 ± 3.74 | 24.46 ± 4.45 *,※ | 27.78 ± 4.99 **,※※,†† | 21.87 ± 4.15 | < 0.001 |
| SV (mL) | 65.35 ± 10.17 | 67.06 ± 12.46 | 80.75 ± 14.14 **,※※,†† | 64.55 ± 9.93 | < 0.001 |
| SVi (mL/m2) | 36.64 ± 6.42 | 36.75 ± 6.68 | 43.31 ± 6.74 **,※※,†† | 35.95 ± 5.01 | < 0.001 |
| LVEF | 62.87 ± 3.49 | 62.23 ± 3.42 | 61.24 ± 1.18 | 62.26 ± 3.01 | 0.387 |
| RWT | 0.36 ± 0.04 | 0.38 ± 0.05 *,※ | 0.40 ± 0.06 **,※※,† | 0.35 ± 0.04 | 0.009 |
| LVM (g) | 134.0 (109.4, 176.7) | 159.1 (135.1, 182,5) ** | 224.0 (135.1, 238.8) **,※※,†† | 132.9 (101.8, 143.2) | < 0.001 |
| LVMi (g/m2) | 77.5 (65.9, 95.7) | 87.9 (73.1, 99.7) ** | 114.4 (101.2, 129.4) **,※※,†† | 71.8 (56.7, 81.2) | < 0.001 |
| E (cm/s) | 99.4 (89.5, 116.7) | 89.8 (85.4, 99.6) | 67.5 (56.3, 78.9) **,※※,†† | 99.6 (88.6, 112.6) | < 0.001 |
| A (cm/s) | 69.8 (61.5, 78.1) | 75.5 (64.4, 89.9) | 89.6 (81.1, 99.8) **,※※,†† | 75.5 (66.4, 86.7) | < 0.001 |
| E′a (cm/s) | 13.44 ± 1.60 | 13.10 ± 1.97 | 11.39 ± 1.84 **,※※,†† | 12.90 ± 1.57 | < 0.001 |
| E/A ratio | 1.47 ± 0.37 | 1.30 ± 0.44 | 0.84 ± 0.49 **,※※,†† | 1.34 ± 0.35 | 0.001 |
| E/E′a ratio | 7.55 ± 1.20 | 6.93 ± 1.05 | 6.18 ± 1.09 **,※※†† | 7.35 ± 1.04 | < 0.001 |
Note: Normally distributed data are presented as mean ± standard deviation. Non-normally distributed data were expressed as median (IQR). ST, short time; MET, more extended-term; LT, long-term; IVSd, interventricular septum diastolic; LVEDD, left ventricular end-diastlic diameters; LVEDDi, left ventricular end-diastlic diameteers index; PW, left ventricular posterior wall thickness; EDV, left ventricular end-diastlic volume; EDVi, left ventricular end-diastlic volume index; ESV, left ventricular end-systolic volume; ESVi, left ventricular end-systolic volume index; SV, stroke volume; SVi, stroke volume index; LVEF, left ventricular ejection fraction; RWT, Relative wall thickness; LVM, left ventricular mass; LVM, left ventricular mass index; E, early-diastolic velocities; A, late-diastolic atrial filling velocity; E′av, average values early-diastolic velocities by Doppler tissue imaging. * p < 0.05, ** p < 0.01 when compared with controls; ※ p < 0.05, ※※ p < 0.01 when compared with ST; † p < 0.05, †† p < 0.01when compared with MET.
3.3 The changes in left ventricular mechanical function after marathon running by speckle tracking echocardiography
The speckle tracking echocardiographic data before and after the marathon are exhibited in Table 3. Before marathon (as baseline), the LT runners group showed significantly higher absolute values of apical rotation, LV twist, LV torsion and LV UTR, but a significantly lower absolute value of GLS, compared to the other groups (Table 3, p < 0.05). The GLS values were -19.39 ± 2.68, -21.56 ± 2.56, -21.04 ± 2.26 and -21.52 ± 2.35 in LT runners, ST runners, MET runners, and controls, respectively (Table 3). 1 hour after marathon, the absolute value of GCS was significantly lower in the LT runners group compared with the ST and MET runners (Table 3, p < 0.05). LV twist and LV torsion demonstrated similar trends across all 3 amateur marathon runners groups 1 hour after marathon, with no significant differences observed (Table 3, p > 0.05). Compared to baseline measurement, the absolute value of all speckle tracking echocardiographic parameters was decreased 1 hour after marathon in all amateur marathon runners (p < 0.05), and returned to baseline 4 days after marathon, showing no statistical differences before and 4 days after marathon (Table 3). Δ GLS, Δ GCS, Δ Apical rotation, Δ LV twist, Δ LV torsion, and Δ LV UTR were markedly elevated in the LT runners group relative to the ST runners and the MET runners groups (Table 4, p < 0.01). Basal rotation was similar in all 3 amateur marathon runners groups (Table 4). The apical rotation image and basal rotation image and left ventricular torsion diagram (speckle echocardiography) of the amateur marathon runners in the LT group can be seen in Figure 1-3.
Table 3 Speckle tracking echocardiography characteristics of the study population.
| Variable | Amateur marathon runners | Controls (n = 31) | p | ||
|---|---|---|---|---|---|
| ST (n = 23) | MET (n = 32) | LT (n = 30) | |||
| GLS (%) | |||||
| Before marathon | -21.56 ± 2.56 | -21.04 ± 2.26 | -19.39 ± 2.68 **,※※,†† | -21.52 ± 2.35 | < 0.001 |
| 1 hour after marathon | -20.69 ± 2.16 ⁋⁋ | -20.39 ± 1.83 ⁋⁋ | -18.30 ± 2.68 ※※,††,⁋⁋ | NA | 0.006 |
| 4 days after marathon | -21.46 ± 2.06 ▼▼ | -21.31 ± 2.15 ▼▼ | -19.15 ± 2.71 ※※,††,▼▼ | NA | < 0.001 |
| GCS (%) | |||||
| Before marathon | -23.41 ± 2.94 | -23.26 ± 2.56 | -22.56 ± 3.06 | NA | 0.563 |
| 1 hour after marathon | -22.33 ± 2.47 ⁋⁋ | -22.15 ± 2.28 ⁋⁋ | -21.02 ± 2.88※,††,⁋⁋ | NA | < 0.001 |
| 4 days after marathon | -23.18 ± 2.81 ▼▼ | -23.05 ± 2.49 ▼▼ | -22.28 ± 2.55 ▼▼ | NA | 0.293 |
| Apical rotation (°) | |||||
| Before marathon | 7.34 ± 2.11 | 7.60 ± 2.01 | 8.95 ± 2.61 **,※※,†† | 7.33 ± 2.11 | 0.008 |
| 1 hour after marathon | 6.11 ± 1.84 ⁋⁋ | 6.64 ± 1.86 ⁋⁋ | 7.21 ± 1.98 ※,⁋⁋ | NA | 0.104 |
| 4 days after marathon | 6.83 ± 1.88 ▼▼ | 7.59 ± 1.93 ▼▼ | 8.61 ± 2.23 ※※,†,⁋,▼▼ | NA | 0.009 |
| Basal rotation (°) | |||||
| Before marathon | -5.71 ± 1.65 | -5.84 ± 1.96 | -6.53 ± 2.23 | -5.61 ± 1.98 | 0.294 |
| 1 hour after marathon | -5.13 ± 1.56 ⁋⁋ | -5.27 ± 1.92 ⁋⁋ | -5.56 ± 1.95 ⁋⁋ | NA | 0.802 |
| 4 days after marathon | -5.60 ± 1.71 ▼▼ | -5.77 ± 1.85▼▼ | -6.38 ± 2.05 ▼▼ | NA | 0.393 |
| LV twist (°) | |||||
| Before marathon | 13.05 ± 2.79 | 13.44 ± 3.69 | 15.56 ± 3.83 *,※※,† | 12.85 ± 3.14 | 0.029 |
| 1 hour after marathon | 11.23 ± 2.50 ⁋⁋ | 11.92 ± 3.32 ⁋⁋ | 12.67 ± 3.37 ⁋⁋ | NA | 0.281 |
| 4 days after marathon | 12.97 ± 2.67 ▼▼ | 13.36 ± 3.5 ▼▼ | 14.88 ± 3.71 ※※,†,⁋⁋,▼▼ | NA | 0.033 |
| LV torsion (°/cm) | |||||
| Before marathon | 1.65 ± 0.38 | 1.69 ± 0.50 | 1.99 ± 0.55 *,※,†† | 1.66 ± 0.48 | 0.036 |
| 1 hour after marathon | 1.43 ± 0.35 ⁋⁋ | 1.51 ± 0.47 ⁋⁋ | 1.63 ± 0.48 ⁋⁋ | NA | 0.259 |
| 4 days after marathon | 1.60 ± 0.37 ▼▼ | 1.68 ± 0.49 ▼▼ | 1.91 ± 0.58 ※,†,⁋⁋,▼▼ | NA | 0.033 |
| LV UTR (°/s) | |||||
| Before marathon | 58.70 (49.15, 72.10) | 67.80 (58.00, 79.82) | 98.82 (61.33, 115.50) **,※※,† | 58.50 (47.50, 71.25) | 0.001 |
| 1 hour after marathon | 52.10 (44.10, 67.08) ⁋⁋ | 65.50 (56.70, 73.80) ⁋⁋ | 82.20 (56.32, 110.30) ※,†,⁋⁋ | NA | 0.024 |
| 4 days after marathon | 56.70 (47.15, 70.19) | 66.70( 56.60, 73.88) ▼▼ | 96.30 (59.30, 112.40) ※※,†,▼ | NA | 0.005 |
Note: Normally distributed data are presented as mean ± standard deviation. Non-normally distributed data were expressed as median (IQR). ST, short time; MET, more extended-term; LT, long-term; GLS, global longitudinal strain; GCS, global circumferential strain; LV, left ventricular; UTR, untwisting rate. * p < 0.05, ** p < 0.01 when compared with controls. ※ p < 0.05, ※※ p < 0.01 when compared with ST. † p < 0.05, †† p < 0.01 when compared with MET. In intra-group comparison: ⁋ p < 0.05, ⁋⁋ p < 0.01 when compared to before marathon. ▼ p < 0.05, ▼▼ p < 0.01 when compared with 1 hour after marathon.
Table 4 The change of spcekle tracking echocardiography characteristics of the amateur marathon runners.
| Variable | Amateur marathon runners | p | ||
|---|---|---|---|---|
| ST (n = 23) | MET (n = 32) | LT (n = 30) | ||
| Δ GLS (%) | 4.21 (1.95, 7.47) | 4.26 (2.88, 8.65) | 7.40 (4.23, 11.23)※,† | 0.042 |
| Δ GCS (%) | 3.93 (2.76 ,5.41) | 4.45 (3.52, 6.95) | 6.72 (4.37, 10.55)※,† | 0.014 |
| Δ Apical rotation (%) | 11.82 (7.37, 16.67) | 13.89 (9.71, 22.31) | 18.86 (13.23, 23.59)※※,† | 0.016 |
| Δ Basal rotation (%) | 10.23 ± 6.67 | 10.33 ± 8.67 | 15.86 ± 11.15 | 0.078 |
| Δ LV twist (%) | 11.64 ± 6.12 | 13.91 ± 5.98 | 18.45 ± 6.90※※,† | < 0.001 |
| Δ LV torsion (%) | 12.56 ± 5.97 | 14.68 ± 6.92 | 18.65 ± 7.05 ※※,† | < 0.001 |
| Δ LV UTR (%) | 11.37 (8.04, 14.07) | 11.96 (10.10, 15.03) | 15.56 (10.69, 18.60) ※,† | 0.021 |
Note: Normally distributed data are presented as mean ± standard deviation. Non-normally distributed data were expressed as median (IQR). ST, short time; MET, more extended-term; LT, long-term; GLS, global longitudinal strain; GCS, global circumferential strain; LV, left ventricular; UTR, untwisting rate. ※ p < 0.05, ※※ p < 0.01 when compared with ST. † p < 0.05, †† p < 0.01 when compared with MET.
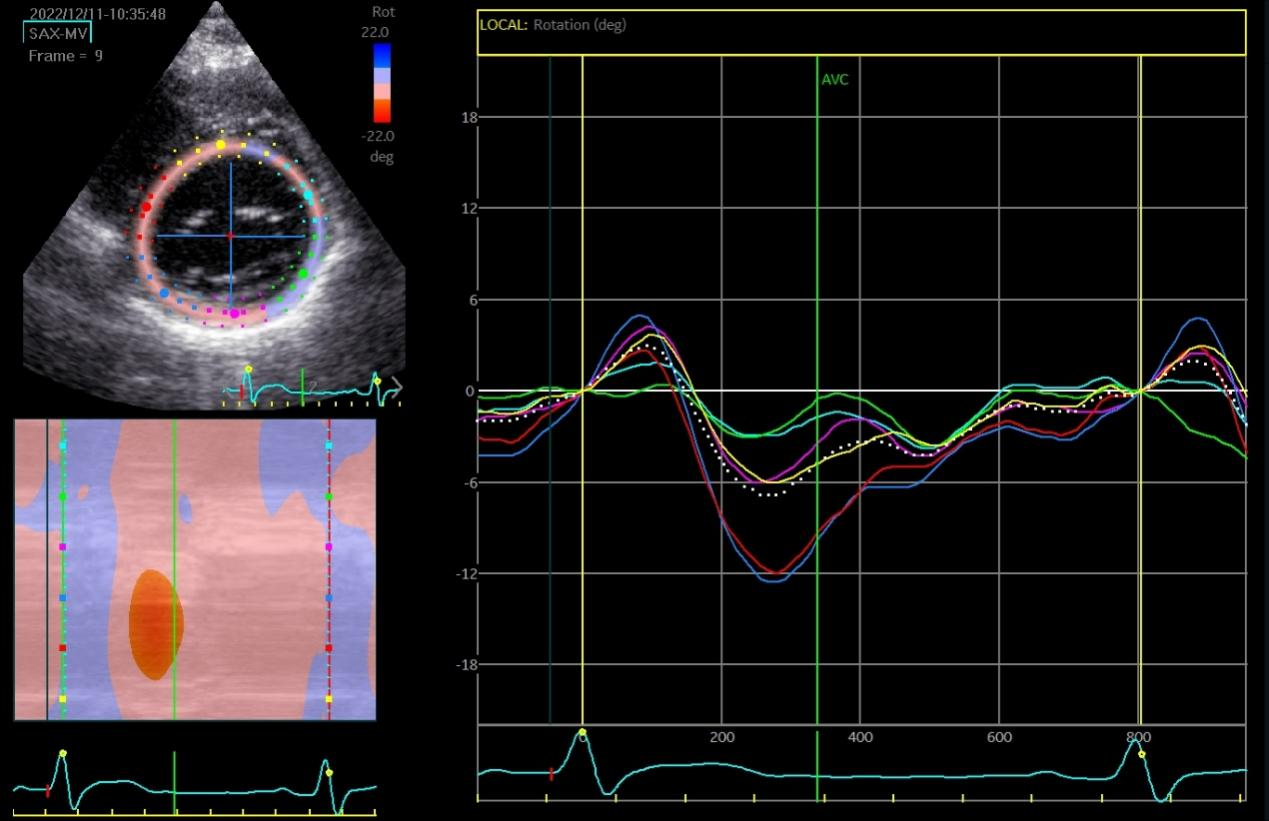
Figure 1 Apical rotation image (speckle tracking echocardiographic) in LT group amateur marathon runners. LT, long-term.
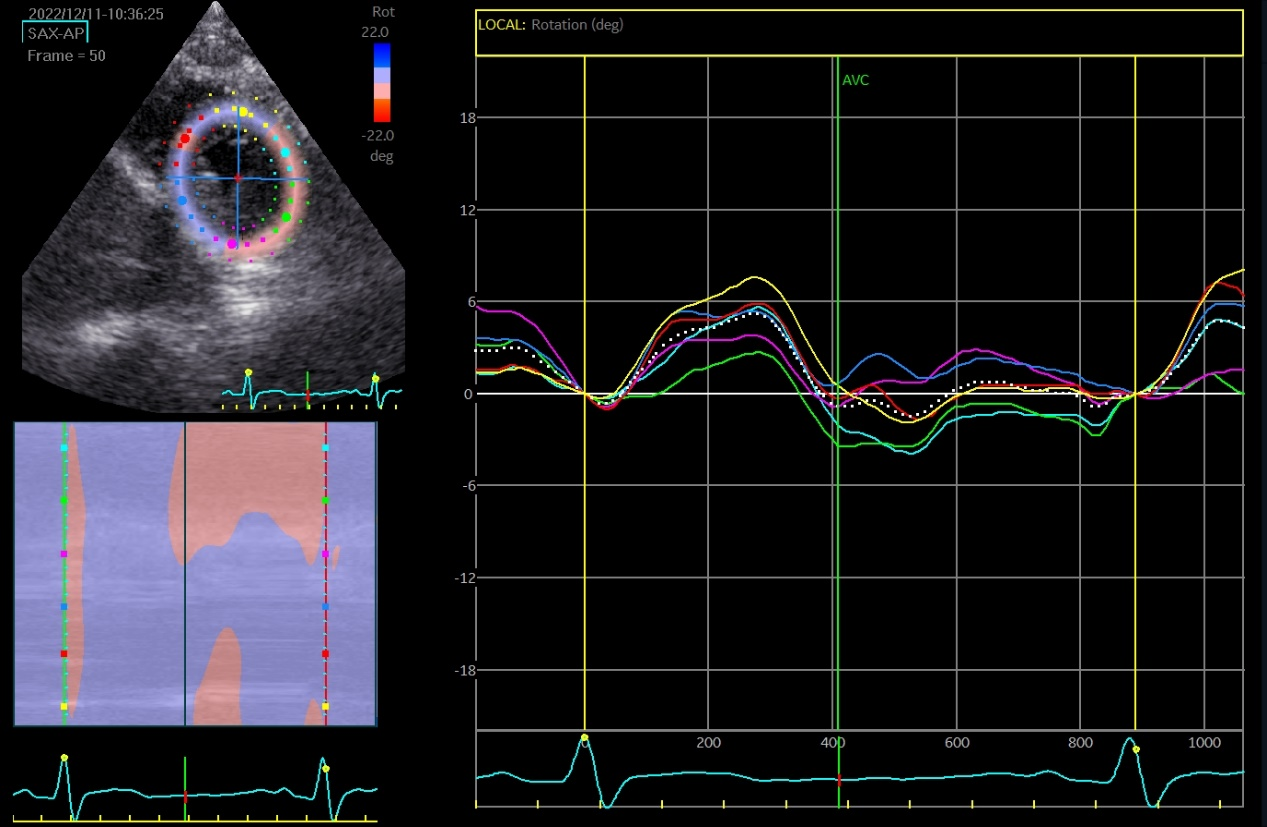
Figure 2 Basal rotation image (speckle tracking echocardiographic) in LT group amateur marathon runners. LT, long-term.
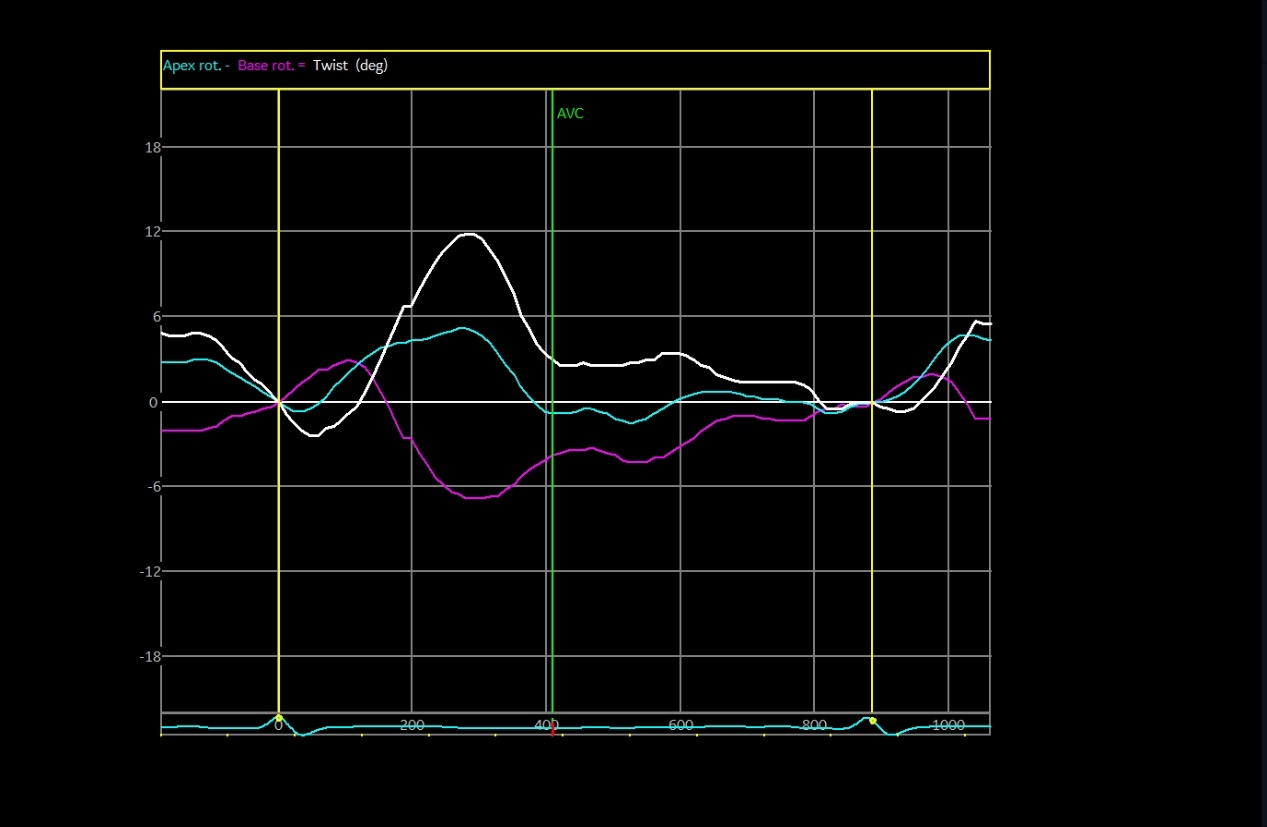
Figure 3 Left ventricular twist image (speckle tracking echocardiographic) in LT group amateur marathon runners. LT, long-term.
3.4 Intra- and Inter-observer reproducibility
The intra-class correlation coefficient for intra- and inter-observer reproducibility was 0.89 (95% confidence interval (CI) 0.86 to 0.92) and 0.91 (95% CI 0.88 to 0.95) for GLS, 0.88 (95% CI 0.85 to 0.93) and 0.90 (95% CI 0.86 to 0.95) for GCS, 0.87 (95% CI 0.85 to 0.92) and 0.90 (95% CI 0.86 to 0.92) for apical rotation, 0.86 (95% CI 0.83 to 0.90) and 0.83 (95% CI 0.80 to 0.88) for LV twist, and 0.86 (95% CI 0.84 to 0.92) and 0.88 (95% CI 0.86 to 0.91) for peak LV UTR (Table 5).
Table 5 Intra-class correlation coefficient for intra- and inter-observer reproducibility of left ventricular mechanical parameters.
| Parameter | Intra-observer reproducibility | 95% CI | Inter-observer reproducibility | 95% CI |
|---|---|---|---|---|
| GLS | 0.89 | 0.86 - 0.92 | 0.91 | 0.88 - 0.95 |
| GCS | 0.88 | 0.85 - 0.93 | 0.90 | 0.86 - 0.95 |
| Apical rotation | 0.87 | 0.85 - 0.92 | 0.90 | 0.86 - 0.92 |
| LV twist | 0.86 | 0.83 - 0.90 | 0.83 | 0.80 - 0.88 |
| Peak LV UTR | 0.86 | 0.84 - 0.92 | 0.88 | 0.86 - 0.91 |
Note: CI, confidence interval
3.5 Investigation on Traditional Chinese Medicine (TCM) constitution types of amateur marathon athletes
The analysis of TCM body constitutions revealed that the Pinghezhi (balanced constitution) was the most common type among amateur marathon runners across all age groups. However, its distribution varied with age: it was least prevalent in the 33-41 years age group (21.88%) and most prevalent in the 42-50 years age group (37.50%). The trends of other constitutions also varied significantly with age (Table 6).
Table 6 Distribution of TCM constitution types among amateur marathon athletes of different ages [n (%)].
| Constitutional type | 24-32 (years) (n = 23) | 33-41 (years) (n = 32) | 42-50 (years) (n = 30) |
|---|---|---|---|
| Pinghe Constitution | 7 (30.43) | 7 (21.88) | 12 (37.50) |
| Qi Deficiency Constitution | 3 (13.04) | 4 (12.50) | 4 (13.33) |
| Yang Deficiency Constitution | 4 (17.39) | 5 (15.63) | 3 (10.00) |
| Yin Deficiency Constitution | 1 (4.35) | 3 (9.38) | 1 (3.33) |
| Phlegm-Dampness Constitution | 1 (4.35) | 2 (6.25) | 2 (6.67) |
| Dampness-Heat Constitution | 4 (17.39) | 3 (9.38) | 1 (3.33) |
| Blood Stasis Constitution | 1 (4.35) | 4 (12.50) | 4 (13.33) |
| Qi Depression Constitution | 1 (4.35) | 1 (3.13) | 1 (3.33) |
| Inherited Special Constitution | 1 (4.35) | 3 (9.38) | 1 (3.33) |
Note: CI, confidence interval
4 Discussion
Whether participation in and preparation for a full marathon represents a healthy promotion of increased regular physical activity or a potentially cardiotoxic dose of endurance exercise remains debatable. Marathon runners develop different cardiac adaptations according to their exercise intensity (static or dynamic). In dynamic exercise, maximal cardiac output, the product of SV and heart rate, is increased from 5-6 L/min to 40 L/min on average. Increases in heart rate and SV (to a lesser degree) contribute to cardiac output augmentation during acute exercise in runners. Cardiac chamber enlargement and the accompanying ability to generate a large SV are direct results of exercise training and are the cardiovascular hallmarks of the endurance-trained athlete [16]. In this study, we evaluated the morphological and functional characteristics of LV remodeling in different amateur marathon runners based on time of completing marathon. The major findings of our study were: (1) At baseline, LV structure and systolic function in novice amateur marathon runners did not change after training for and completing a first marathon, despite having a lower heart rate than health subjects; (2) Lower absolute GLS of LV,, along with greater apical rotation and LV twist, were observed in runners who had long-term marathon participation; (3) A brief decease in LV systolic function was observed post-marathon across all groups, with a more pronounced reduction in long-term amateur marathon runners.
Dilation of the LV, either with or without concomitant mild thickening of LV walls, may exist in the endurance-trained athlete. Balanced LV chamber dilation and wall thickening are common among competitive athletes engaged in endurance sports [17]. Our study demonstrated higher LV diameters, wall thicknesses, and LVM in MET and LT runners with an abnormal LV hypertrophy (defined by increased LVM and a relative wall thickness < 0.42). LVEF was similar among groups and within normal ranges, reflecting both systolic functions. GLS of LV was less negative in the LT runners. It is well-established that the 'physiological' hypertrophy in marathon runner shows increased LVM and normal organization of cardiac structure with no increase in collagen content, whereas hypertrophic cardiomyopathy is associated with structural collagen accumulation, myofibrillar changes, and potential diastolic and systolic dysfunction [18,19]. In our study, GLS of LT runners was lower compared with controls, whereas GCS was not significantly different, consistent with previous studies that compared various types of LV systolic deformation and found that the behaviour of longitudinal systolic function of subendocardial fibers can distinguish trained athletes from patients with hypertrophic cardiomyopathy or systemic hypertension [20].
LV twist and torsion make great impacts upon ventricular systolic and diastolic function. The torsional recoil during early diastolic and isovolumic relaxation releases the potential energy, resulting in storage of twist during ejection [21]. Physiological variables such as contractility, preload, and afterload alter the extent of LV twist and torsion. LV twist is greater with higher preload; for example, with ESV held constant, higher EDV of LV produce higher LV twist [22]. Like changes in loading condition, increasing contractility promotes LV twist. In the intact circulation, changes in contractility are often accompanied by altered loading conditions for changing the twist mechanics. Recent studies have shown that LV twist and rotation might be increased in endurance-trained athletes [23,24]. In our study, although LV basal rotation did not significantly differ among various stages of amateur marathon runners or between marathon runners and controls, LV apical rotation, twist, torsion, and UTR showed an increased trend in the LT runners. While the mechanisms of our findings remain speculative, factors including loading conditions, LV geometry, and cellular composition warrant consideration. Long endurance exercise training results in significant cardiac chamber enlargement and blood volume expansion. Reportedly, LV mechanics are preload-dependent [25]. As such, vascular volume expansion may contribute to the enhanced LV twist and UTR that are related to long endurance exercise training. Functional studies on cardiac trabecular muscles and cardiomyocytes revealed greater calcium sensitivity, rate of force production and loaded shortening velocities in exercised rats [26]. Besides, changes in cellular protein expression may in part explain our study results. Cardiac compliance is mainly regulated by the giant elastic protein, titin, which can be used to determine the elastic, contractile and signaling properties of cardiac and skeletal muscles, and plays an integral role in the storage and release of potential elastic energy during systole and diastole. Studies have confirmed that titin modifications after long-term exercise are potential adaptive mechanisms that contribute to improved cardiac compliance and favorable performance in the long endurance exercise training [27,28]. Collectively, cardiac titin may be one of the causes for the observed changes in LV twist mechanics.
Our study supports the growing evidence that running a marathon can result in a significant and transient decrease in LV systolic function. Moreover, our results strongly demonstrated an alteration of the twisting mechanics of the LV post-marathon, characterized by a decrease in basal and apical rotations and consequently LV twist. To date, several factors have been proposed to play independent or synergistic roles in this phenomenon: (1) post-exercise alterations in loading and heart rate; (2) subclinical levels of cardiomyocyte damage, in which myocardial damage/stunning has largely participated, and myocardial damage/stunning involved descriptive data with the release/appearance of cardiac-specific biomarkers of myocyte damage alongside changes in LV function; (3) β-adrenergic desensitization (many studies have suggested that the increased circulating catecholamines during prolonged exercise may result in decreased cardiac β-receptor function or desensitisation via downregulation and/or uncoupling with the subsequent reduction in LV inotropic and chronotropic responses, leading to LV dysfunction); (4) serial or parallel ventricular interaction (data suggested a relatively remarkable elevation of pulmonary artery pressure and therefore a disproportionately higher stroke work load in the right ventricular compared to the LV during prolonged marathon). The thin-walled right ventricular myocardium may not sustain contractile force against an elevated afterload for a prolonged period, leading to reduced right ventricular contractility, impaired downstream or “serially” left atrial preload, decreased LV filling and subsequently affected LV systolic function.
Our study demonstrates a clear gradient of cardiac adaptation positively correlated with training duration. The Long-Term group, which largely overlaps with the older runners in our cohort (42-50 years), exhibited the most profound remodeling. This finding is further contextualized by the TCM constitution analysis, which identified the highest prevalence of the robust Pinghezhi in this same age group. This correlation suggests a potential bidirectional relationship: long-term endurance training may promote a more balanced physiological state, and/or individuals with an innate balanced constitution may be more likely to adhere to and thrive under the demands of long-term marathon training, thus achieving superior cardiac adaptations. The lower prevalence of Pinghezhi in the middle-aged group (33-41 years), who likely face peak career and family demands, may hint at the impact of lifestyle stress on constitutional balance and its potential interplay with training response.
One of the limitations in this study is the relatively small sample size, causing relatively weak statistical power. Moreover, we could not fully control marathon runners' environment, including meals, drinks, sleep, training intensity, and time limit for marathon completion to be uniform. LV twist and torsion measured by two-dimensional speckle tracking are not actually global twist and torsion, as they were derived from only two short-axis views (at the apical level and mitral valve). The development of three-dimensional speckle tracking might resolve this issue. Follow-up studies may be needed for a better understanding of the clinical impact of these results.
5 Conclusion
We have employed a prospective, longitudinal study design to evaluate the LV twist functions of amateur marathon runners. Our findings demonstrate that the duration of endurance training plays a critical role in modulating cardiac structure and function in amateur marathon runners. Long-term training is associated with more significant left ventricular remodeling and enhanced myocardial mechanic. However, acute changes post-marathon, particularly in myocardial deformation parameters, suggest transient functional stress even in well-trained individuals. These results highlight the importance of individualized training strategies and regular cardiac monitoring in endurance athletes to ensure both performance optimization and cardiovascular safety.
Back Matter
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Substantial contributions to conception and design: H.Z. Data acquisition, data analysis and interpretation: L.L. and Y.Y. Drafting the article or critically revising it for important intellectual content: Q.W. and Z.Y. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors.
Ethics Approval and Consent to Participate
This study was approved by the Ethics Committee of our hospital. Written informed consent was obtained from the runners.
Funding
This study was supported by the Medical and Health Science & Technology Program of Gongshu District, Hangzhou (Grant No. B202310).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- D'Silva A, Bhuva AN, van Zalen J, et al. Cardiovascular remodeling experienced by real-world, unsupervised, young novice marathon runners. Frontiers in Physiology 2020; 11: 232.
- Scheer V, Costa RJS, Doutreleau S, et al. Recommendations on youth participation in ultra-endurance running events: a consensus statement. Sports Medicine 2021; 51(6): 1123-1135.
- Roberts WO, Stovitz SD. Incidence of sudden cardiac death in Minnesota high school athletes 1993-2012 screened with a standardized pre-participation evaluation. Journal of the American College of Cardiology 2013; 62(14): 1298-1301.
- Pelliccia A, Caselli S, Sharma S, et al. European Association of Preventive Cardiology (EAPC) and European Association of Cardiovascular Imaging (EACVI) joint position statement: recommendations for the indication and interpretation of cardiovascular imaging in the evaluation of the athlete's heart. European Heart Journal 2018; 39(21): 1949-1969.
- Pelliccia A, Quattrini FM, Cavarretta E, et al. Physiologic and clinical features of the paralympic athlete's heart. JAMA Cardiology 2021; 6(1): 30-39.
- Maron BJ, Levine BD, Washington RL, et al. Eligibility and disqualification recommendations for competitive athletes with cardiovascular abnormalities: Task Force 2: preparticipation screening for cardiovascular disease in competitive athletes: a scientific statement from the American Heart Association and American College of Cardiology. Circulation 2015; 132(22): e267-272.
- Buckberg GD, Hoffman JI, Coghlan HC, et al. Ventricular structure-function relations in health and disease: Part I. The normal heart. European Journal of Cardio-Thoracic Surgery 2015; 47(4): 587-601.
- Domenech-Ximenos B, Sanz-de la Garza M, Sepulveda-Martinez Á, et al. Assessment of myocardial deformation with CMR: a comparison with ultrasound speckle tracking. European Radiology 2021; 31(10): 7242-7250.
- Sengupta SP, Mahure C, Mungulmare K, et al. Myocardial fatigue in recreational marathon runners: a speckle-tracking echocardiography study. Indian Heart Journal 2018; 70 Suppl 3(Suppl 3): S229-S234.
- Lord RN, Utomi V, Oxborough DL, et al. Left ventricular function and mechanics following prolonged endurance exercise: an update and meta-analysis with insights from novel techniques. European Journal of Applied Physiology 2018; 118(7): 1291-1299.
- Oxborough D, Whyte G, Wilson M, et al. A depression in left ventricular diastolic filling following prolonged strenuous exercise is associated with changes in left atrial mechanics. Journal of the American Society of Echocardiography 2010; 23(9): 968-976.
- Lang RM, Badano LP, Mor-Avi V, et al. Recommendations for cardiac chamber quantification by echocardiography in adults: an update from the American Society of Echocardiography and the European Association of Cardiovascular Imaging. Journal of the American Society of Echocardiography 2015; 28(1): 1-39.e14.
- Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification. European Journal of Echocardiography 2006; 7(2): 79-108.
- Gherbesi E, Gianstefani S, Angeli F, et al. Myocardial strain of the left ventricle by speckle tracking echocardiography: from physics to clinical practice. Echocardiography 2024; 41(1): e15753.
- Hjertaas JJ, Einarsen E, Gerdts E, et al. Impact of aortic valve stenosis on myocardial deformation in different left ventricular levels: a three-dimensional speckle tracking echocardiography study. Echocardiography 2023; 40(10): 1028-1039.
- Dawkins T. Evidence of functional remodelling of the right and left ventricle in athletes. Cardiff Metropolitan University: Cardiff, UK, 2021.
- King G, Wood MJ. The heart of the endurance athlete assessed by echocardiography and its modalities: "embracing the delicate balance". Current Cardiology Reports 2013; 15(8): 383.
- Liu S, Laghzali O, Shalikar S, et al. Cardiac MRI strain as an early indicator of myocardial dysfunction in hypertrophic cardiomyopathy. International Journal of Molecular Sciences 2025; 26(4): 1407.
- Whyte G, George K, Shave R, et al. Impact of marathon running on cardiac structure and function in recreational runners. Clinical Science 2005; 108(1): 73-80.
- Vinereanu D, Florescu N, Sculthorpe N, et al. Differentiation between pathologic and physiologic left ventricular hypertrophy by tissue Doppler assessment of long-axis function in patients with hypertrophic cardiomyopathy or systemic hypertension and in athletes. The American Journal of Cardiology 2001; 88(1): 53-58.
- Singh RB, Elkilany GN, Fedaco J, et al. Evolution of the natural history of myocardial twist and diastolic dysfunction as cardiac dysfunction. In Pathophysiology, Risk Factors, and Management of Chronic Heart Failure; Elsevier: Cambridge, MA, USA, 2024; pp.65-77.
- Sengupta PP, Tajik AJ, Chandrasekaran K, et al. Twist mechanics of the left ventricle: principles and application. JACC: Cardiovascular Imaging 2008; 1(3): 366-376.
- Unnithan VB, Beaumont A, Rowland T, et al. Left ventricular responses during exercise in highly trained youth athletes: echocardiographic insights on function and adaptation. Journal of Cardiovascular Development and Disease 2022; 9(12): 438.
- Perkins D. The influence of maturation on left ventricular remodelling and haematological adaptation with endurance training. Cardiff Metropolitan University: Cardiff, UK, 2022.
- Balthazaar SJT, Nightingale TE, Alrashidi AA, et al. Effects of exercise interventions on cardiac structure, function, and mechanics in individuals with chronic motor-complete spinal cord injury: an exploratory randomized clinical trial. Topics in Spinal Cord Injury Rehabilitation 2025; 31(2): 62-75.
- Boldt KR, Rios JL, Joumaa V, et al. Force properties of skinned cardiac muscle following increasing volumes of aerobic exercise in rats. Journal of Applied Physiology 2018; 125(2): 495-503.
- Kellermayer D, Kiss B, Tordai H, et al. Increased expression of N2BA titin corresponds to more compliant myofibrils in athlete's heart. International Journal of Molecular Sciences 2021; 22(20): 11050.
- Stebbings GK, Williams AG, Herbert AJ, et al. TTN genotype is associated with fascicle length and marathon running performance. Scandinavian Journal of Medicine & Science in Sports 2018; 28(2): 400-406.