Main Text
1 Introduction
1.1 Traditional uses and modern significance of Polygonum multiflorum Thunb.
Polygonum multiflorum Thunb. (PM), commonly known as Heshouwu, has been a cornerstone of traditional Chinese medicine (TCM) for over a millennium. Historically, it has been prescribed for its purported anti-aging, hepatoprotective, and neuroprotective properties, primarily attributed to its ability to "nourish the liver and kidneys" and "blacken hair" [1]. The root of PM is classified into two forms in TCM: raw (Shengshouwu) and processed (Zhishouwu), with the latter being subjected to the traditional "nine-steaming and nine-sun-drying" (Jiuzheng jiushai, JZS) to enhance efficacy and reduce toxicity [2].
Modern pharmacological studies have identified key bioactive constituents in PM, including: Stilbenes (e.g., 2,3,5,4'-tetrahydroxystilbene-2-O-β-D-glucoside, TSG), a potent antioxidant linked to anti-aging effects [3,4]; Anthraquinones (e.g., emodin, physcion), which exhibit laxative and anti-inflammatory properties but may contribute to hepatotoxicity [5]; Polysaccharides, known for immunomodulatory and hypoglycemic activities [6].
Despite its therapeutic reputation, PM has faced scrutiny due to increasing reports of drug-induced liver injury (DILI), raising questions about its safety profile [7].
1.2 Hepatotoxicity controversy: balancing efficacy and safety
The dual nature of PM—both as a revered tonic and a potential hepatotoxin—has sparked debate in the scientific community. Clinical case studies have associated PM consumption with elevated liver enzymes, hepatitis, and even acute liver failure, particularly with unprocessed or improperly prepared roots [8]. For instance, a meta-analysis by [9] identified 320 cases of PM-related DILI in China between 2010–2019, with raw PM accounting for 85% of incidents. Recent studies using 1H NMR-based metabolomics have revealed that raw PM induces hepatotoxicity in a non-linear manner, with low-dose raw PM causing the most severe oxidative stress and mitochondrial dysfunction, while medium and high doses exhibit attenuated effects due to potential hepatoprotective mechanisms. This paradoxical dose-response relationship underscores the complexity of PM's biological effects, where oxidative stress and inflammatory responses (e.g., elevated TNF-α and COX-2) coexist with adaptive anti-oxidative responses (e.g., upregulation of HO-1) [10].
The toxicity is hypothesized to stem from multiple mechanisms. Anthraquinone derivatives (e.g., free emodin) may induce mitochondrial dysfunction and oxidative stress [11], as evidenced by disrupted energy metabolism (e.g., TCA cycle impairment) and amino acid metabolism in hepatocytes [10]. Additionally, recent research highlights the role of bile acid (BA) and bilirubin (BIL) metabolism disruption in PM-induced liver injury [12]. Wang et al. demonstrated that PM blocks both BIL and BA metabolic pathways by downregulating key transporters (e.g., NTCP, OATP1B1/3, MRP2, BSEP) and the metabolic enzyme UGT1A1, leading to cholestasis and hyperbilirubinemia. Notably, free anthraquinones are identified as primary hepatotoxic components, while TSG exhibits dose-dependent effects, demonstrating antioxidant and neuroprotective activities at therapeutic doses but potentially exacerbating BA metabolic disruption at excessive concentrations [13].
Critically, the processing method significantly alters toxicity. The traditional JZS method reduces free anthraquinones by up to 90% while preserving TSG [14], corroborating findings that processed PM exhibits markedly lower hepatotoxicity in both animal models and clinical settings. Metabolomic analyses further confirm that JZS processing transforms hepatotoxic free anthraquinones into safer polymeric forms and generates protective metabolites like 5-HMF, which mitigate oxidative stress and inflammation. These scientific validations align with traditional claims of "detoxification through processing" and highlight the critical importance of standardized preparation methods in ensuring PM's safety profile.
1.3 Geographic variability and processing: key determinants of quality
The chemical composition of PM is highly sensitive to geographic origin and cultivation practices, with climatic conditions playing a significant role—for example, Guizhou Province's high-altitude regions produce PM with 20–30% higher TSG content compared to lowland counterparts. Soil micronutrients, such as selenium-rich soils, further influence quality by enhancing polysaccharide synthesis [15]. Cultivation practices also impact composition, as wild PM typically contains higher TSG but lower emodin levels than cultivated varieties [16], while harvest timing is critical, with 3–4-year-old roots optimizing bioactive yields [17]. Despite these complexities, current quality standards, such as those in the Chinese Pharmacopoeia [18], remain narrowly focused on TSG and emodin, overlooking other pharmacologically relevant markers [19].
This review systematically evaluates the impact of geographic origin on PM phytochemical profile while deciphering how JZS modulates bioactive constituents and mitigates toxicity. By integrating ethnopharmacology, metabolomics, and toxicology, the study proposes evidence-based strategies for quality control and safe clinical use, aiming to bridge traditional knowledge with modern scientific evidence and address critical gaps identified in recent critiques.
2. Variations in bioactive constituents of Polygonum multiflorum Thunb. across different geographic origins
2.1 Classification and pharmacological functions of key bioactive compounds
PM contains a complex array of bioactive constituents, which can be broadly categorized into three major classes based on their chemical structures and pharmacological activities: anthraquinones, stilbenes, and polysaccharides, along with minor constituents such as tannins and trace elements [16].
2.1.1 Anthraquinones
Anthraquinones are among the most studied bioactive compounds in PM, with emodin, physcion, and chrysophanol being the predominant derivatives [20]. These compounds exhibit laxative effects by stimulating colonic motility, making PM a potential therapeutic agent for constipation [21]. Beyond their gastrointestinal effects, anthraquinones demonstrate anti-inflammatory and anticancer properties through the inhibition of NF-κB and MAPK signaling pathways [22,23].
However, anthraquinones also present potential hepatotoxic risks, particularly in their unbound forms. Emodin, for instance, has been shown to induce oxidative stress and mitochondrial dysfunction, contributing to liver injury in some cases [24]. A study by Kang et al. further confirmed that excessive intake of PM extracts rich in free anthraquinones could lead to DILI, emphasizing the need for proper dosage control and processing methods to mitigate toxicity [25].
2.1.2 Stilbenes
The predominant stilbene in PM, TSG, is a key bioactive compound responsible for many of the herb’s anti-aging and neuroprotective effects [26,27]. TSG exerts potent antioxidant activity by activating the Nrf2/ARE pathway, which enhances cellular defense mechanisms against oxidative stress [3]. Recent studies have highlighted TSG's potential in neurodegenerative disease treatment. For example, a study demonstrated that TSG improves cognitive function in Alzheimer's disease (AD) models by reducing β-amyloid plaque accumulation and tau protein hyperphosphorylation [28]. Additionally, TSG has been shown to protect dopaminergic neurons in Parkinson's disease models, suggesting its broad applicability in age-related neurological disorders [29].
Beyond its neuroprotective and anti-aging properties, TSG exhibits significant pharmacological activities in treating a wide range of chronic diseases. Modern pharmacological studies confirm that TSG demonstrates remarkable anti-inflammatory effects by regulating NF-κB, AMPK/Nrf2, and NLRP3 inflammasome pathways, making it a potential therapeutic agent for inflammatory diseases such as colitis and neuroinflammation [30]. In cardiovascular diseases, TSG protects endothelial cells, inhibits vascular smooth muscle cell proliferation, and attenuates atherosclerosis through TGF-β/Smad, eNOS/NO, and RhoA/ROCK signaling pathways [30]. TSG also shows hepatoprotective effects in non-alcoholic fatty liver disease (NAFLD) and alcoholic hepatic steatosis by modulating lipid metabolism and gut microbiota. Furthermore, TSG promotes osteoblast differentiation and inhibits osteoclastogenesis, suggesting its potential in osteoporosis treatment [31]. Its antidepressant-like effects are linked to the enhancement of the hippocampal BDNF system, while its renoprotective role in diabetic nephropathy involves the inhibition of oxidative stress and inflammation [32].
2.1.3 Polysaccharides
PM-derived polysaccharides exhibit immunomodulatory and anti-fatigue effects, making them valuable for enhancing immune function [33]. These polysaccharides activate macrophages and dendritic cells, promoting cytokine release and improving immune surveillance.
PM polysaccharides also have demonstrated hypoglycemic effects in diabetic models by enhancing insulin sensitivity and reducing oxidative stress [34]. A study further revealed that PM polysaccharides could protect against colitis by modulating gut microbiota, highlighting their potential in gastrointestinal health [35].
Moreover, recent studies have uncovered novel mechanisms by which PM polysaccharides exert anti-aging effects. Research indicates that these polysaccharides can modulate the P53/P21 pathway, a critical regulator of cellular senescence, thereby delaying age-related functional decline. Additionally, PM polysaccharides have been shown to regulate amino acid metabolism, particularly the biosynthesis of serine, glycine, and methionine, which are essential for maintaining cellular homeostasis and mitigating oxidative stress during aging [36]. These findings suggest that PM polysaccharides may serve as a multifaceted intervention for aging, targeting both genomic stability and metabolic reprogramming.
2.1.4 Minor constituents
In addition to the major bioactive compounds, PM contains tannins, flavonoids, and trace elements (zinc, selenium), which contribute to its overall pharmacological profile [2]. Tannins exhibit antioxidant and anti-inflammatory properties, while trace elements like selenium enhance cellular antioxidant defenses [37].
Recent research has also identified novel minor compounds, such as PMF-1 (a unique flavonoid), which shows anti-tumor activity in breast cancer cell lines [38]. These findings suggest that PM's minor constituents may play a more significant role in its therapeutic effects than previously recognized.
2.2 Impact of geographic origin on phytochemical profiles
2.2.1 Climatic factors
The accumulation of bioactive compounds in PM exhibits distinct climatic dependencies. For TSG, regions with high-altitude environments and intense ultraviolet (UV) radiation, such as Guizhou, exhibits superior accumulation due to UV-induced activation of the phenylpropanoid pathway [39]. Moderate temperatures (15-25 ℃) and consistently high humidity further support this biosynthetic route, as seen in the mountainous areas of Sichuan and Yunnan. Conversely, arid conditions like Gansu tend to suppress TSG synthesis.
In contrast, anthraquinones like emodin thrive in regions with significant diurnal temperature fluctuations (e.g., Sichuan Basin), where daytime heat and cooler nights stimulate oxidative enzyme activity [40]. Temperate climates with stable seasonal variations tend to promote a balanced production of both compound classes, while tropical conditions may lead to metabolic shifts favoring either stilbenes or anthraquinones, but rarely both, highlighting the need for region-specific cultivation strategies to optimize desired phytochemical profiles.
2.2.2 Soil composition
Soil properties further modulate the geographic variability of PM's chemical composition. Selenium-rich soils in Guizhou correlate with elevated polysaccharide content [41], while phosphorus-deficient soils in Guangdong are associated with increased anthraquinone production [42]. In addition, studies have found the purple soils of Sichuan basin, known for their good drainage and mineral content, often yield PM with relatively high emodin levels. The mountainous regions of Guizhou typically feature yellow-brown soils rich in humus and micronutrients, which appear conducive to TSG biosynthesis [43]. However, saline-alkali soils in some northern cultivation areas have been associated with reduced TSG accumulation, likely due to impaired nutrient uptake and physiological stress on the plants [44].
2.2.3 Regional comparisons and quality implications
We purchased 50 batches of PM from different geographical origins through Hong Kong markets and determined their TSG and emodin contents using high-performance liquid chromatography (HPLC) analysis (Figure 1). Our analysis revealed notable deviations from theoretical expectations. While high-altitude regions like Guizhou were anticipated to show superior TSG content, the highest median levels (868.62 mg/L) unexpectedly occurred in samples from Hong Kong. This discrepancy may reflect: (1) limited samples size (n = 1) from Guizhou were tested; (2) unaccounted cultivation practices in Hong Kong samples, such as controlled shading that mimic high-UV conditions; (3) genetic adaptation of local cultivars to tropical environments; (4) post-harvest processing variations affecting compound stability. Similarly, Sichuan's samples showed the highest anthraquinone levels (6.85 mg/L), consistent with its continental climate, but the median anthraquinones content of 0.00 mg/L in Guangdong samples contradicts expectations for phosphorus-deficient soils. Potential explanations include: (1) the diverse sourcing of Guangdong samples resulted in considerable quality variation; (2) undisclosed pre-processing methods; (3) soil depletion despite historical classification. These findings underscore the complex interplay between genotype, environment, and agricultural practice in determining final product quality. Table 1 below summarizes key regional differences in PM's bioactive compounds, including measured content variations and their expected trends based on environmental factors, highlighting the need for region-specific quality standard.
Table 1 Regional variations in key bioactive compounds of Polygonum multiflorum Thunb.
| Region | TSG (mg/L, Median) | Emodin (mg/L, Median) | Climate profile | Dominant soil factor | Expected component trends |
|---|---|---|---|---|---|
| Hong Kong * | 868.62 | 0.18 | Tropical, high humidity | Urban (limited data) | TSG ↑↑ (due to optimized UV exposure in shaded cultivation); Polysaccharides → (insufficient soil data) |
| Sichuan | 614.56 | 6.85 | Temperate, large diurnal swing | Loamy, neutral pH | Emodin ↑↑ (significant diurnal variation promotes oxidation); TSG ↑ (moderate altitude supports accumulation) |
| Guangdong † | 410.15 | 0.00 | Subtropical, monsoon | Low phosphorus | Emodin ↑ (theoretical expectation, but the diverse sourcing exhibits quality heterogeneity); Trace elements ↓ (low phosphorus limits synthesis) |
| Guangxi | 40.25 | 0.00 | High humidity, stable temps | Sandy, acidic | Polysaccharides ↑ (acidic sandy soil promotes accumulation); TSG ↓ (low UV exposure inhibits synthesis) |
| Guizhou | 119.70 | 0.00 | High-altitude, strong UV | Selenium-rich | TSG ↑↑ (UV activates phenylpropanoid pathway, but insufficient samples were tested); Polysaccharides ↑↑ (selenium synergy); Emodin ↓ (altitude inhibits oxidation) |
| Gansu | 237.06 | 0.06 | Continental, arid | Calcareous, alkaline | Minerals ↑↑ (alkaline soil enriches Ca/Zn); TSG → (aridity may limit accumulation) |
Note:
1. Data obtained from 50 commercially available PM batches in Hong Kong markets but different origins; The cultivation types (wild/cultivated) were not specified by suppliers.
2. * TSG levels in Hong Kong exceed typical high-altitude expectations; † Emodin below expectation (see discussion in 2.2.3).
3. ↑↑, significant increase; ↑, moderate increase; →, no significant change; ↓, decrease.
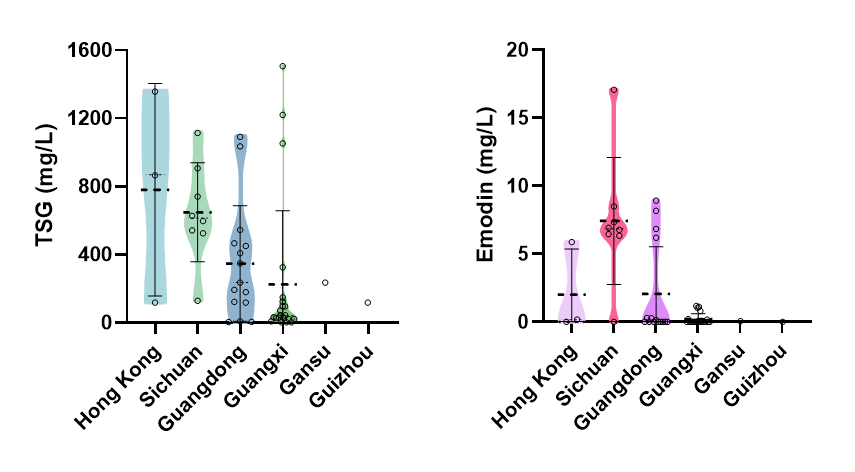
Figure 1 Comparative analysis of TSG and Emodin content in 50 batches of PM from different origins purchased in Hong Kong (self-generated unpublished data).
2.3 Wild vs. cultivated Polygonum multiflorum Thunb.: a chemical comparison
Wild and cultivated PM exhibit notable differences in their chemical composition due to distinct growth conditions and cultivation practices. Wild PM demonstrates superior quality in certain aspects, containing higher TSG levels (2–3%) as a result of natural stress adaptation, along with lower emodin content (0.05 mg/g), which may reduce potential hepatotoxicity risks. In contrast, cultivated PM presents a yield-quality trade-off, where conventional fertilization (e.g., N-P-K) increases biomass but leaves the content of harmful elements unchanged. Additionally, harvest timing significantly influences bioactive compound accumulation, with 3-year-old roots maximizing TSG content, while 4-year-old roots achieve peak polysaccharide levels [45-47]. These findings highlight the critical role of cultivation methods in determining the medicinal value of PM.
3. Effects of nine-steaming and nine-sun-drying processing on bioactive constituents of Polygonum multiflorum Thunb.
3.1 Traditional theory and scientific basis of processing
The ancient Chinese processing method of JZS for PM represents a remarkable integration of empirical knowledge and philosophical principles. Rooted in Taoist alchemical traditions dating back to the Tang Dynasty (618-907 AD), this elaborate processing technique was developed to transform the crude herb's "cold and descending" nature into a "warm and ascending" property suitable for tonifying liver and kidney functions according to TCM theory [48]. Historical texts such as the "Ben Cao Gang Mu" (Compendium of Materia Medica, 1596 AD) describe this process as essential for "converting toxicity into efficacy", particularly for achieving the herb's renowned hair-blackening and longevity-promoting effects [49].
Modern scientific investigations have systematically validated these traditional claims through advanced analytical techniques. Comparative metabolomic studies reveal that JZS processing induces profound changes in PM's phytochemical profile, affecting over 120 identified compounds [50]. The alternating steaming (typically 4-6 hours at 100-105 ℃) and sun-drying (8-12 hours under natural sunlight) create unique conditions for chemical transformations that cannot be replicated by simple heating or drying alone [51]. This cyclic processing generates specific Maillard reaction products, oxidative conjugates, and glycoside hydrolysis patterns that define the processed herb's pharmacological properties.
3.2 Dynamic changes of chemical constituents during processing
The traditional JZS processing method induces profound chemical transformations in PM, which are critical for its detoxification and efficacy enhancement. This section systematically examines these changes, focusing on anthraquinones, stilbenes, polysaccharides, and other minor constituents, supported by recent scientific evidence.
3.2.1 Anthraquinone transformation pathways
The JZS processing method induces profound chemical transformations in anthraquinones, the primary hepatotoxic constituents of PM. During the initial steaming cycles, bound anthraquinone glycosides (e.g., emodin-8-O-β-D-glucoside, EG) undergo enzymatic hydrolysis by heat-stable β-glucosidases, releasing free aglycones such as emodin and physcion [52]. This hydrolysis follows first-order kinetics, with rate constants of 0.15–0.25 h-1 at 100–105 ℃ [53]. Subsequent cycles promote oxidative polymerization and glycosylation of these free anthraquinones, forming oligomeric derivatives (e.g., emodin dimers and trimers) with reduced hepatotoxicity but retained bioactivity [54]. Advanced liquid chromatography-mass spectrometry (LC-MS) analyses reveal that JZS reduces free anthraquinones by 85–92%, while bound forms increase by 300–500% due to re-glycosylation [55]. These transformations align with traditional claims of "detoxification" as polymerized anthraquinones exhibit lower mitochondrial toxicity and ROS generation in HepG2 cells [56].
3.2.2 Stilbene glycoside stabilization
Contrary to the labile nature of anthraquinones, the key bioactive stilbene TSG demonstrates remarkable stability during JZS processing. Studies attribute this to three mechanisms: (1) formation of protective complexes with melanoidins generated via Maillard reactions during steaming [57]; (2) pH-dependent isomerization to stable conformers under cyclic thermal stress [58]; and (3) microencapsulation within starch-protein matrices, which enhances solubility and bioavailability [59]. Notably, TSG content increases by 250% after JZS, likely due to matrix modification and release from bound forms (Table 2). This stabilization is critical for PM's neuroprotective effects, as TSG's antioxidant activity via Nrf2/ARE pathway activation remains intact [60].
3.2.3 Polysaccharide degradation and functional modulation
JZS processing selectively degrades high-molecular-weight polysaccharides (50 kDa → 28 kDa) through controlled hydrolysis, improving immunomodulatory activity [61]. The reduced polysaccharide size enhances macrophage activation and cytokine release (e.g., IL-6 and TNF-α) by 40–60% compared to raw PM [62]. This degradation is temperature-dependent, with optimal effects observed at 105°C during later steaming cycles [63].
3.2.4 Generation of process-specific metabolites
JZS generates unique metabolites absent in raw PM, such as 5-hydroxymethylfurfural (5-HMF), a Maillard reaction product with neuroprotective properties [64]. 5-HMF levels increase >100-fold after processing (Table 2) and correlate with PM’s clinical efficacy in cognitive impairment [65]. Other novel compounds include oligomeric anthraquinones and glycosylated emodin derivatives, which contribute to reduced toxicity [66].
3.2.5 Kinetic and thermodynamic insights
The alternating steaming and sun-drying cycles create dynamic conditions for chemical reactions. Steaming phases (4–6 hours at 100–105 ℃) drive hydrolysis and polymerization, while sun-drying (8–12 hours at 30–50% humidity) facilitates oxidative condensation [67]. This cyclic process cannot be replicated by continuous heating, as demonstrated by differential scanning calorimetry (DSC) studies [68].
Table 2 Comprehensive chemical changes during JZS processing.
| Compound class | Raw PM content | After JZS processing | Change trend | Mechanism | Biological significance |
|---|---|---|---|---|---|
| Free anthraquinones | 1.2-1.8 mg/g | 0.1-0.3 mg/g | ↓ 85-92% | Hydrolysis → polymerization | Toxicity reduction |
| Bound anthraquinones | 0.5-0.8 mg/g | 2.1-2.8 mg/g | ↑ 300-500% | Glycosylation of free anthraquinones | Improved bioavailability |
| TSG | 12-15% | 38-42% | ↑ 250% | Matrix modification | Enhanced efficacy |
| 5-HMF | < 0.01 mg/g | 1.2-2.0 mg/g | > 100× | Maillard reactions | New neuroactivity |
| Polysaccharide | 50 kDa | 28 kDa | ↓ 44% | Controlled degradation | Improved immunomodulation |
Note: ↑, increase; ↓, decrease; →, indicates a transformation process.
3.3 Optimization of processing parameters and modern adaptations
The traditional JZS process has undergone extensive scientific scrutiny to identify optimal parameters and potential modernization approaches while maintaining its essential character. Contemporary research has precisely quantified the effects of processing variables, establishing that four hours of steaming per cycle at temperatures gradually increasing from 100 to 105 ℃ across cycles provides the ideal balance between transformation and degradation of key components [69]. The drying conditions, particularly the maintenance of 30-50% relative humidity during sun-drying phases, have been shown to be superior to artificial drying methods for preserving certain chemical profiles. While nine complete cycles remain the gold standard for full transformation, studies indicate that seven cycles can achieve approximately 85% of the desired benefits with significantly reduced time and energy expenditure. Modern technological adaptations have focused on standardizing this traditionally variable process while preserving its core detoxification and efficacy-enhancing principles. Innovations such as far-infrared assisted drying maintain the cyclic thermal stress essential for anthraquinone polumerization, pulsed vacuum steaming ensures uniform hydrolysis of glycosides akin to traditional steaming, and online NIR monitoring validates the achievement of key chemical markers (e.g., TSG stabilization and 5-HMF generation) that define traditional efficacy and safety. High-humidity hot air impingement steaming systems that achieve 95% batch consistency while replicating the Maillard reactions and oxidative condensation observed in sun-drying phases [70]. These optimizations adhere to the Taoist alchemical philosophy of "converting toxicity into efficacy" by ensuring the reduction of free anthraquinones (85–92%) and preservation of TSG bioavailability, as empirically validated in historical texts. However, complete replacement of traditional methods with artificial processes consistently alters critical quality attributes, underscoring the necessity of hybrid approaches that integrate modern precision with traditional cyclic alternation of steaming and sun-drying [71]. These findings highlight the delicate balance required when modernizing traditional processing methods to maintain their unique pharmacological advantages while improving reproducibility and efficiency.
3.4 Clinical correlations and safety profile
The chemical changes induced by proper JZS processing directly correlate with measurable improvements in clinical outcomes and safety profiles. Processed PM demonstrates an 80% reduction in hepatotoxicity incidence compared to raw materials [72], validating traditional claims about toxicity reduction through processing. Clinically, optimal JZS samples show superior efficacy in multiple applications, including an 89% response rate for alopecia treatment compared to 62% for raw preparations [73], significant improvement in mild cognitive impairment as measured by MMSE scores, and better outcomes for age-related fatigue. These clinical benefits are consistently associated with the specific chemical profile produced by authentic JZS processing, particularly the balanced ratio of transformed anthraquinones to preserved TSG and the presence of process-specific bioactive compounds. Ongoing challenges in the field include establishing universal standards for "authentic" JZS among commercial manufacturers, developing robust processing-property-activity relationships for quality control purposes, and determining the appropriate degree of modernization that preserves the essential qualities of traditional processing while addressing contemporary production needs. The accumulated evidence strongly supports the continued use and refinement of JZS processing as a vital step in preparing PM for clinical use, combining ancient wisdom with modern scientific understanding to optimize both safety and therapeutic efficacy.
4. Quality control and standardization: current challenges and solutions
4.1 Limitations of existing quality standards
The standardization of PM aligns with the broader evolution of TCM standardization, which has progressed from initial awareness (1980s) to rapid system-building (2006–2020) and now emphasizes high-quality development (post-2021) [74]. However, similar to TCM standardization challenges, PM faces unbalanced standard coverage (e.g., overemphasis on TSG/emodin) and insufficient implementation of advanced techniques like metabolomics, despite their potential to bridge traditional and modern quality control.
The current quality control measures for PM, as outlined in pharmacopoeias such as the Chinese Pharmacopoeia (ChP 2020), primarily focus on the quantification of a limited number of markers, notably TSG and emodin. While these compounds are pharmacologically significant, this narrow approach fails to capture the full complexity of PM's phytochemical profile, which includes over 120 identified constituents with potential therapeutic or toxicological relevance [75]. For instance, the ChP 2020 mandates a minimum TSG content of 1.0% for raw PM and 0.7% for processed PM, alongside an emodin limit of 0.1%, but overlooks critical components such as polysaccharides, oligomeric anthraquinones, and process-specific metabolites like 5-hydroxymethylfurfural (5-HMF). This oversimplification has led to inconsistencies in clinical outcomes, as products meeting pharmacopoeial standards may still exhibit variable efficacy or safety due to unregulated components.
In addition to intrinsic hepatotoxic components, exogenous contaminants such as heavy metals (e.g., cadmium, lead), pesticide residues, and mycotoxins (e.g., aflatoxins from improper storage) may further compromise PM safety. These contaminants often originate from environmental pollution, agricultural practices, or post-harvest handling. For instance, PM grown in industrial areas or soils with high metal bioavailability may accumulate toxic elements, while improper drying or storage can promote mold growth. Although the Chinese Pharmacopoeia sets limits for some contaminants (e.g., heavy metals ≤20 ppm), enforcement varies regionally, and comprehensive screening for pesticides or mycotoxins remains rare. Future standards should integrate contaminant monitoring with metabolomic profiling to address both endogenous and exogenous risks.
Moreover, the existing standards do not account for the influence of geographic origin or processing methods on PM's chemical composition. For example, PM from Guizhou typically contains higher TSG levels (up to 6–7%) compared to other regions, yet the current standards apply uniformly across all sources [76]. Similarly, the ChP lacks specific criteria to distinguish between properly and inadequately processed PM, despite evidence that incomplete JZS cycles may retain hepatotoxic anthraquinones. These gaps underscore the need for a more comprehensive and nuanced quality assessment framework.
4.2 Toward a multidimensional quality evaluation system
Recent advancements in analytical technologies and systems biology have paved the way for more holistic quality control strategies. A promising approach involves the integration of multiple analytical methods to evaluate PM's chemical integrity, efficacy, and safety. For instance, fingerprinting techniques such as LC-MS can profile a broader range of bioactive compounds, including stilbenes, anthraquinones, and polysaccharides [77]. Metabolomic studies have further identified unique biomarkers for authentic JZS-processed PM, such as specific ratios of free to bound anthraquinones and the presence of 5-HMF, which correlates with proper processing [78].
In addition to chemical profiling, bioactivity assays could serve as complementary tools for quality assessment. For example, antioxidant capacity tests (e.g., DPPH and ORAC assays) and anti-inflammatory models (e.g., RAW 264.7 macrophage assays) have been proposed to evaluate PM's functional properties. Such assays align with the traditional use of PM and may better predict clinical efficacy than single-marker quantification. Furthermore, toxicological screening using in vitro hepatocyte models or in vivo zebrafish assays could help identify batches with residual hepatotoxicity. [79]
4.3 The role of fingerprint analysis and metabolomics
Fingerprint analysis has emerged as a powerful tool for PM quality control, enabling the simultaneous detection of multiple constituents and their relative abundances. Studies have demonstrated that HPLC fingerprints of properly processed PM exhibit characteristic peaks corresponding to TSG, transformed anthraquinones, and Maillard reaction products (e.g., 5-HMF) [80], which are absent or diminished in substandard products. By applying chemometric methods such as principal component analysis (PCA) or hierarchical cluster analysis (HCA), researchers can differentiate PM samples based on origin, processing method, or adulteration.
Metabolomics, particularly untargeted LC-MS-based approaches, offers even greater resolution by capturing subtle variations in PM's chemical profile. For instance, a recent study identified 15 process-specific metabolites in JZS-treated PM, including novel oligomeric anthraquinones and glycosylated derivatives of emodin, which contribute to its reduced toxicity and enhanced efficacy [78]. These findings suggest that metabolomic signatures could serve as reliable markers for authentication and standardization.
4.4 Integrating traditional knowledge and modern technology
The modernization of PM quality control must also respect and incorporate traditional knowledge. For centuries, TCM practitioners have relied on organoleptic properties including color, texture, and taste to assess PM quality. Interestingly, scientific studies have validated some of these empirical indicators. For example, the blackening of PM during JZS processing correlates with the formation of melanoidins and 5-HMF [57], which are associated with reduced toxicity and improved bioactivity. Similarly, the "sticky" texture of properly processed PM reflects polysaccharide degradation and matrix modification, which enhance solubility and absorption.
Emerging technologies such as artificial intelligence (AI) and machine learning (ML) are now being explored to bridge traditional and modern quality assessment methods. For instance, convolutional neural networks (CNNs) trained on images of PM slices can predict processing quality with over 90% accuracy by recognizing visual cues traditionally used by herbalists [81]. Portable near-infrared (NIR) devices coupled with ML algorithms have also been developed for rapid, on-site quality screening, enabling real-time decision-making in production facilities [82].
4.5 Regulatory and industrial implications
The implementation of advanced quality control measures faces practical challenges, including cost, technical expertise, and regulatory acceptance. However, the growing demand for standardized herbal products in global markets necessitates such improvements. Pilot studies have demonstrated the feasibility of adopting fingerprinting and metabolomic approaches in industrial settings. For example, a collaborative project between academia and PM manufacturers in Guizhou successfully reduced batch-to-batch variability by 40% through LC-MS-based quality monitoring [77].
Regulatory agencies must also evolve to accommodate these advancements. The development of region-specific monographs or "quality tiers" could address geographic variability, while process-specific standards (e.g., minimum cycles for JZS) would ensure proper preparation. International harmonization efforts, such as the WHO's guidelines for herbal medicines, could further facilitate the adoption of these methods globally [83].5. Toxicological evaluation and safe use of Polygonum multiflorum Thunb.
5.1 Hepatotoxicity mechanisms and constituent-toxicity relationships
The hepatotoxicity associated with PM has been a focal point of modern research, particularly due to increasing reports of DILI. Studies have identified anthraquinones, especially free emodin and physcion, as primary contributors to liver damage through multiple pathways. These compounds induce mitochondrial dysfunction by uncoupling oxidative phosphorylation, leading to ATP depletion and reactive oxygen species (ROS) overproduction [84]. Additionally, emodin metabolites form covalent bonds with cellular proteins, triggering immune-mediated hepatotoxicity [85,86]. Notably, the unprocessed root (Shengshouwu) exhibits higher toxicity due to its elevated free anthraquinone content, while properly processed PM (Zhishouwu) shows significantly reduced risks, as the traditional JZS method converts 85–92% of free anthraquinones into less toxic polymerized forms [2,87].
Recent metabolomic studies have further elucidated the role of TSG in toxicity. While TSG is generally considered safe at low doses, high concentrations may paradoxically exacerbate oxidative stress by depleting glutathione reserves [30]. This dual role underscores the importance of dose optimization in clinical applications.
5.2 Evidence from preclinical and clinical studies
Animal models have provided critical insights into PM's safety profile. In rats, prolonged administration of raw PM (0.3125∼0.625 g/kg/day for 28 days) resulted in elevated ALT/AST levels and histopathological changes, including hepatocellular necrosis [88,89]. In contrast, JZS-processed PM at equivalent doses showed no significant hepatotoxicity, corroborating traditional claims of detoxification [72]. Cell-based studies using HepG2 cells further demonstrated that processed PM extracts reduced ROS generation by 40-60% compared to raw extracts [90].Clinical data highlight the importance of preparation and dosage. A meta-analysis of 25,927 DILI cases in China (2012-2016) revealed that 26.81% involved TCM, often consumed as self-prepared decoctions exceeding recommended doses [9]. Conversely, standardized processed PM formulations, such as those in TCM patent medicines (e.g., Shouwu Pian), showed a lower incidence of adverse effects [91].
5.3 Strategies for risk mitigation and safe clinical practice
To minimize the hepatotoxicity risks associated with PM, evidence-based strategies emphasize the importance of standardized processing, dose optimization, and personalized approaches. Strict adherence to traditional JZS protocols is critical, as this method ensures the conversion of hepatotoxic free anthraquinones into safer polymerized forms while preserving the therapeutic efficacy of key compounds like TSG. Clinical trials have identified a safe daily dose range of 3–6 g for processed PM, with the addition of hepatoprotective adjuvants such as Glycyrrhiza uralensis recommended for long-term use to further mitigate potential liver injury [73].
Emerging research also highlights the role of genetic predispositions in individual susceptibility to PM-induced liver damage. For instance, patients carrying HLA-B * 35 : 01 alleles may require tailored dosing regimens or alternative therapies to avoid adverse effects [92]. Integrating these strategies—standardized processing, precise dosing, and genetic screening—can significantly enhance the safety profile of PM, ensuring its continued use in both traditional and modern medical practices.
6. Future research frontiers
6.1 Molecular mechanisms of processing efficacy
The traditional JZS method for PM has been empirically validated for reducing hepatotoxicity, but its molecular mechanisms remain incompletely understood. Future research should focus on elucidating the enzymatic and non-enzymatic pathways involved in JZS-induced chemical transformations. For instance, the role of heat-stable β-glucosidases in anthraquinone hydrolysis during steaming cycles warrants investigation [71]. Advanced techniques like cryo-electron microscopy (cryo-EM) could visualize structural changes in bioactive compounds, such as the polymerization of free anthraquinones into safer oligomeric forms [93,94]. Additionally, metabolomic studies could identify novel process-specific metabolites, such as Maillard reaction products (e.g., 5-HMF), which may contribute to PM's detoxification and enhanced bioactivity [95].
6.2 AI-driven quality control
The variability in PM's phytochemical profile due to geographic and processing factors necessitates advanced quality control methods. Artificial intelligence (AI) and machine learning (ML) offer promising solutions. For example, CNNs trained on HPLC or LC-MS datasets could predict optimal harvesting times or processing endpoints, reducing human error and improving batch consistency [96]. Blockchain technology could further enhance traceability by documenting each step of PM production, from cultivation to clinical use, ensuring transparency and adherence to standards [97,98]. These technologies could bridge the gap between traditional knowledge and modern scientific validation, facilitating global acceptance of PM-based therapies.
6.3 Precision medicine approaches
Individual variability in response to PM highlights the need for personalized treatment strategies. Future research should explore biomarkers for toxicity susceptibility, such as genetic polymorphisms (e.g., HLA-B * 35 : 01 alleles) or metabolomic signatures [92,99]. Collaborative efforts between TCM practitioners and modern pharmacologists could integrate empirical knowledge with scientific validation, enabling tailored dosing regimens. For instance, patients with genetic predispositions to hepatotoxicity might benefit from lower doses of processed PM with black soybean juice or adjuvants like Glycyrrhiza uralensis [69,100]. Clinical trials incorporating these approaches could establish evidence-based guidelines for PM's safe and effective use.
7 Conclusion
This comprehensive review systematically elucidates the critical interplay between geographic origin, cultivation practices, and processing methods in determining the pharmacological profile and safety of PM. The evidence underscores that bioactive constituents such as anthraquinones, stilbenes, and polysaccharides exhibit significant variability across regions, with climatic and edaphic factors playing pivotal roles. For instance, PM exposed to strong UV radiation consistently demonstrates superior TSG content, while diurnal temperature variations favor anthraquinone accumulation. The traditional JZS process emerges as a cornerstone for detoxification, reducing free anthraquinones, while preserving TSG bioavailability. Modern analytical techniques, including metabolomics and fingerprinting, validate these transformations and highlight the limitations of current pharmacopoeial standards, which overlook key markers like 5-HMF and oligomeric anthraquinones.
To ensure PM's safe clinical application, future efforts must prioritize the development of multidimensional quality control systems integrating chemical, bioactivity, and toxicity assessments. Regional-specific standards and advanced technologies (e.g., AI-driven traceability) could address batch variability. Furthermore, interdisciplinary collaboration is essential to bridge traditional knowledge with contemporary science, optimizing PM's therapeutic potential while mitigating risks. As global demand for herbal medicines grows, this synthesis of ethnopharmacology and modern research provides a robust framework for standardizing PM production and fostering evidence-based use.
Back Matter
Abbreviation
5-HMF: 5-(Hydroxymethyl)furfural; AI: Artificial Intelligence; ALT: Alanine Aminotransferase; AST: Aspartate Aminotransferase; BA: Bile Acid; BIL: Bilirubin; CNN: Convolutional Neural Network; cryo-EM: Cryo-Electron Microscopy; DILI: Drug-Induced Liver Injury; DSC: Differential Scanning Calorimetry; EG: Emodin-8-O-β-D-glucoside; HCA: Hierarchical Cluster Analysis; HPLC: High-Performance Liquid Chromatography; JZS: Jiuzheng jiushai (nine-steaming and nine-sun-drying); LC-MS: Liquid Chromatography-Mass Spectrometry; ML: Machine Learning; NIR: Near-infrared; N-P-K: Nitrogen-Phosphorus-Potassium; PCA: Principal Component Analysis; PM: Polygonum multiflorum Thunb. (Heshouwu); ROS: Reactive Oxygen Species; TCM: Traditional Chinese Medicine; TSG: 2,3,5,4′-tetrahydroxystilbene-2-O-β-D-glucoside; WHO: World Health Organization
Acknowledgements
The author would like to thank Mr. Keith Wong, Ms Cindy Lee, and Mr. Freddy Tsang for their technical support.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Substantial contributions to conception and design: Z.F. Data acquisition, data analysis and interpretation: H.Y., Y.T.C, Y.F. Drafting the article or critically revising it for important intellectual content: N.W. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors.
Ethics Approval and Consent to Participate
No ethical approval was required for this review.
Funding
This study was supported by the Chinese Medicine Development Fund, Hong Kong, China (No. 19B2/001A_R1).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- Lin L, Ni B, Lin H, et al. Traditional usages, botany, phytochemistry, pharmacology and toxicology of Polygonum multiflorum Thunb.: a review. Journal of Ethnopharmacology 2015; 159: 158-183.
- Qian J, Feng CH, Wu ZY et al. Phytochemistry, pharmacology, toxicology and detoxification of Polygonum multiflorum Thunb.: a comprehensive review. Frontiers in Pharmacology 2024; 15: 1427019.
- Wu TY, Lin JN, Luo ZY, et al. 2,3,4',5-Tetrahydroxystilbene-2-O-beta-D-Glucoside (THSG) Activates the Nrf2 Antioxidant Pathway and Attenuates Oxidative Stress-Induced Cell Death in Mouse Cochlear UB/OC-2 Cells. Biomolecules 2020; 10(3): 465.
- Zhu C, Li J, Tang W, et al. 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-D-glucoside (TSG) from Polygonum multiflorum Thunb.: A Systematic Review on Anti-Aging. International Journal of Molecular Sciences 2025; 26(7): 3381.
- Liu Y, Wang W, Sun M, et al. Polygonum multiflorum-Induced Liver Injury: Clinical Characteristics, Risk Factors, Material Basis, Action Mechanism and Current Challenges. Frontiers in Pharmacology 2019; 10: 1467.
- Zhang Q, Xu Y, Lv J, et al. Structure characterization of two functional polysaccharides from Polygonum multiflorum and its immunomodulatory. International Journal of Biological Macromolecules 2018; 113: 195-204.
- Frenzel C, Teschke R. Herbal Hepatotoxicity: Clinical Characteristics and Listing Compilation. International Journal of Molecular Sciences 2016; 17(5): 588.
- Jung KA, Min HJ, Yoo SS, et al. Drug-Induced Liver Injury: Twenty Five Cases of Acute Hepatitis Following Ingestion of Polygonum multiflorum Thunb. Gut and Liver 2011; 5(4): 493-499.
- Yang Y, Ge FL, Tang JF, et al. A review of herb-induced liver injury in mainland china. Frontiers in Pharmacology 2022; 13: 813073.
- Ruan LY, Li MH, Xing YX, et al. Hepatotoxicity and hepatoprotection of Polygonum multiflorum Thund. as two sides of the same biological coin. Journal of Ethnopharmacology 2019; 230: 81-94.
- Qu K, Shen NY, Xu XS, et al. Emodin induces human T cell apoptosis in vitro by ROS-mediated endoplasmic reticulum stress and mitochondrial dysfunction. Acta Pharmacologica Sinica 2013; 34(9): 1217-1228.
- Jiang J, Wang Q, Wu Q, et al. Angel or devil: the dual roles of 2,3,5,4'-tetrahydroxystilbene-2-O-beta-D-glucopyr-anoside in the development of liver injury based on integrating pharmacological techniques: a systematic review. Frontiers in Pharmacology 2025; 16: 1523713.
- Wang Q, Wen HR, Ma SC, et al. Polygonum multiflorum Thunb. Induces hepatotoxicity in SD rats and hepatocyte spheroids by Disrupting the metabolism of bilirubin and bile acid. Journal of Ethnopharmacology 2022; 296: 115461.
- Li WF, Wang Y, Qiu CX, et al. Processing-induced reduction in dianthrones content and toxicity of Polygonum multiflorum: Insights from ultra-high performance liquid chromatography triple quadrupole mass spectrometry analysis and toxicological assessment. Animal Models and Experimental Medicine 2025; 8(4): 685-695.
- Ye D, Yang L, Zhou M. Spatiotemporal Variation in Ecosystem Health and Its Driving Factors in Guizhou Province. Land 2023; 12(7): 1439.
- Liu Y, Wang Q, Yang JB, et al. Polygonum multiflorum Thunb.: A Review on Chemical Analysis, Processing Mechanism, Quality Evaluation, and Hepatotoxicity. Frontiers in Pharmacology 2018; 9: 364.
- Pu WT, Yu YF, Shi XX, et al. The Effect of Seasonal and Annual Variation on the Quality of Polygonatum Cyrtonema Hua Rhizomes. Plants 2024; 13(24): 3459.
- Chinese Pharmacopoeia Commission. Pharmacopoeia of the People's Republic of China. China Medical Science Press; 2020.
- Liu LX, Wang Y, Zhang Q et al. Quality markers screening of traditional Chinese medicine prescriptions based on the multi-factor analysis strategy: Jin-Zhen oral liquid as a case. Arabian Journal of Chemistry 2024; 17(1): 105433.
- Wang SX, Kong X, Chen N, et al. Hepatotoxic metabolites in Polygoni Multiflori Radix- Comparative toxicology in mice. Frontiers in Pharmacology 2022; 13: 1007284.
- Liu LW. Chronic constipation: current treatment options. Canadian Journal of Gastroenterology 2011; 25(Suppl B): 22B-28B.
- Park SY, Jin ML, Kang NJ, et al. Anti-inflammatory effects of novel polygonum multiflorum compound via inhibiting NF-kappaB/MAPK and upregulating the Nrf2 pathways in LPS-stimulated microglia. Neuroscience Letters 2017; 651: 43-51.
- Xin D, Li H, Zhou S, et al. Effects of Anthraquinones on Immune Responses and Inflammatory Diseases. Molecules 2022; 27(12): 3831.
- Lin LF, Yuan F, Liu YL, et al. Hepatotoxicity and mechanism study of chrysophanol-8-O-glucoside in vitro. Biomedicine & Pharmacotherapy 2019; 120: 109531.
- Kang L, Li D, Jiang X, et al. Hepatotoxicity of the Major Anthraquinones Derived From Polygoni Multiflori Radix Based on Bile Acid Homeostasis. Frontiers in Pharmacology 2022; 13: 878817.
- Gao Y, Li JT, Li JP, et al. Tetrahydroxy stilbene glycoside alleviated inflammatory damage by mitophagy via AMPK related PINK1/Parkin signaling pathway. Biochemical Pharmacology 2020; 177: 113997.
- Zhou L, Hou Y, Yang QD, et al. Tetrahydroxystilbene glucoside improves the learning and memory of amyloid-beta((1)(-)(4)(2))-injected rats and may be connected to synaptic changes in the hippocampus. Canadian Journal of Physiology and Pharmacology 2012; 90(11): 1446-1455.
- Wu WX, Su YZ, Li ZZ et al. Effects of stilbene glycoside on CDK5, MAPK1 and PP1 in Alzheimer's disease mice. Chinese Journal of Gerontology 2022; 42: 2206-2210.
- Huang C, Lin FQ, Wang GQ, et al. Tetrahydroxystilbene Glucoside Produces Neuroprotection against 6-OHDA-Induced Dopamine Neurotoxicity. Oxidative Medicine and Cellular Longevity 2018; 2018: 7927568.
- Wang C, Dai S, Gong LH, et al. A Review of Pharmacology, Toxicity and Pharmacokinetics of 2,3,5,4'-Tetrahydroxystilbene-2-O-beta-D-Glucoside. Frontiers in Pharmacology 2021; 12: 791214.
- Zhang JK, Yang L, Meng GL, et al. Protective effect of tetrahydroxystilbene glucoside against hydrogen peroxide-induced dysfunction and oxidative stress in osteoblastic MC3T3-E1 cells. European Journal of Pharmacology 2012; 689(1-3): 31-37.
- Wang H, Zhao Y, Wang YJ, et al. Antidepressant-like effects of tetrahydroxystilbene glucoside in mice: Involvement of BDNF signaling cascade in the hippocampus. CNS Neuroscience & Therapeutics 2017; 23(7): 627-636.
- Zhang Q, Xu Y, Lv JJ, et al. New utilization of Polygonum multiflorum polysaccharide as macromolecular carrier of 5-fluorouracil for controlled release and immunoprotection. International Journal of Biological Macromolecules 2018; 116: 1310-1316.
- Bi Q, Gu W, Meng FY, et al. Pharmacological and metagenomics evidence of polysaccharide from Polygonum multiflorum in the alleviation of insulin resistance. International Journal of Biological Macromolecules 2020; 164: 1070-1079.
- Chen JQ, Gao YN, Zhang YQ, et al. Research progress in the treatment of inflammatory bowel disease with natural polysaccharides and related structure–activity relationships. Food & Function 2024; 15(11): 5680-5702.
- Fan J, Li YL, Yang S, et al. Two polysaccharides from Polygonum multiflorum Thunb. exert anti-aging by regulating P53/P21 pathway and amino acid metabolism. International Journal of Biological Macromolecules 2025; 306(Pt 3): 141573.
- Andrés CMC, Pérez de la Lastra JM, Juan CA, et al. Antioxidant Metabolism Pathways in Vitamins, Polyphenols, and Selenium: Parallels and Divergences. International Journal of Molecular Sciences 2024; 25(5): 2600.
- You Q, Li D, Ding HY, et al. Pharmacokinetics and Metabolites of 12 Bioactive Polymethoxyflavones in Rat Plasma. Journal of Agricultural and Food Chemistry 2021; 69(43): 12705-12716.
- Wang Y, Mou Y, Lu SL, et al. Polymethoxylated flavonoids in citrus fruits: absorption, metabolism, and anticancer mechanisms against breast cancer. Peer J 2024; 12: e16711.
- Ashrafizadeh M, Zarrabi A, Orouei S et al. Nobiletin in Cancer Therapy: How This Plant Derived-Natural Compound Targets Various Oncogene and Onco-Suppressor Pathways. Biomedicines 2020; 8(5): 110.
- Pan ZP, Feng YF, Wang MZ et al. Geochemical characteristics of soil selenium and evaluation of selenium-rich land resources in Guiyang area. Frontiers in Geochemistry 2023; 1.
- Yang J, Karunarathna SC, Patabendige N, et al. Unveiling the Bioactive Compounds and Therapeutic Potential of Russula: A Comprehensive Review. Journal of Fungi 2025; 11(5): 341.
- Hu CY, Wu W, Zhou XX et al. Spatiotemporal changes in landscape patterns in karst mountainous regions based on the optimal landscape scale: A case study of Guiyang City in Guizhou Province, China. Ecological Indicators 2023; 150: 110211.
- Xing ZH, Bi GH, Li TY, et al. Effect of Harvest Time on Growth and Bioactive Compounds in Salvia miltiorrhiza. Plants 2024; 13(13): 1788.
- Chen H, Gao Y, Ren J. Determination of stilbene glycoside content in wild and cultivated Polygonum multiflorum. Journal of Guiyang College of Traditional Chinese Medicine 2007; (1): 16-17.
- Zou C. Analysis of trace elements in cultivated and wild Polygonum multiflorum in Guizhou. Studies of Trace Elements and Health 2004; (4): 64.
- Feng Y, Yang Y, Cao S, et al. Research progress on the origin, harvesting time, cultivation, processing and quality of He-Shou-Wu. Shandong Chemical Industry 2015; 44(10): 48-50.
- Matos LC, Machado JP, Monteiro FJ, et al. Understanding Traditional Chinese Medicine Therapeutics: An Overview of the Basics and Clinical Applications. Healthcare 2021; 9(3): 257.
- Zhao ZZ. The Original Source of Modern Research on Chinese Medicinal Materials: Bencao Texts. Alternative, Complementary & Integrative Medicine 2017; 3: 1-9.
- Zhou D, Zhao Y, Chen Z et al. Traditional processing increases biological activities of Dendrobium officinale Kimura et. Migo in Southeast Yunnan, China. Scientific Reports 2022; 12(1): 14814.
- Zulfiqar N. The intricacies of the chemistry of solar energy and its innovative implementations in modern technology. SSRN. 2023. Available from: https://ssrn.com/abstract=4550873.
- Yang M, Liu T, Feng WH, et al. Exploration research on hepatotoxic constituents from Polygonum multiflorum root. Zhongguo Zhong Yao Za Zhi 2016; 41(7): 1289-1296.
- Jiang HM, Zheng Z, Wang XL. Kinetic study of methyltriethoxysilane (MTES) hydrolysis by FTIR spectroscopy under different temperatures and solvents. Vibrational Spectroscopy 2008; 46: 1-7.
- Fan PH, Hay, AE, Marston, A, et al. Chemical variability of the invasive neophytes Polygonum cuspidatum Sieb. and Zucc. and Polygonum sachalinensis F. Schmidt ex Maxim. Biochemical Systematics and Ecology 2009; 37: 24-34.
- Zuo WF, Pang Q, Yao LP, et al. Gut microbiota: A magical multifunctional target regulated by medicine food homology species. Journal of Advanced Research 2023; 52: 151-170.
- Taguchi K, Shimada M, Fujii S, et al. Redox Cycling of 9,10-phenanthraquinone to Cause Oxidative Stress Is Terminated through Its Monoglucuronide Conjugation in Human Pulmonary Epithelial A549 Cells. Free Radical Biology and Medicine 2008; 44: 1645-1655.
- Wang HY, Qian H, Yao WR. Melanoidins produced by the Maillard reaction: Structure and biological activity. Food Chemistry 2011; 128: 573-584.
- Nooshkam M, Varidi M, Bashash M. The Maillard reaction products as food-born antioxidant and antibrowning agents in model and real food systems. Food Chemistry 2019; 275: 644-660.
- Shi BS, Guo X, Liu hy, et al. Dissecting Maillard reaction production in fried foods: Formation mechanisms, sensory characteristic attribution, control strategy, and gut homeostasis regulation. Food Chemistry 2024; 438: 137994.
- Kanzler C, Haase PT. Melanoidins Formed by Heterocyclic Maillard Reaction Intermediates via Aldol Reaction and Michael Addition. Journal of Agricultural and Food Chemistry 2020; 68(1): 332-339.
- Jing YS, Zhang SL, Li MS, et al. Research Progress on the Extraction, Structure, and Bioactivities of Polysaccharides from Coriolus versicolor. Foods 2022; 11(14): 2126.
- Murphy EJ, Fehrenbach GW, Abidin IZ, et al. Polysaccharides-Naturally Occurring Immune Modulators. Polymers 2023; 15(10): 2373.
- ElGamal R, Song C, Rayan, AM et al. Thermal Degradation of Bioactive Compounds during Drying Process of Horticultural and Agronomic Products: A Comprehensive Overview. Agronomy 2023; 13(6): 1580.
- Surh YJ, Liem A, Miller JA et al. 5-Sulfooxymethylfurfural as a possible ultimate mutagenic and carcinogenic metabolite of the Maillard reaction product, 5-hydroxymethylfurfural. Carcinogenesis 1994; 15(10): 2375-2377.
- Surh YJ, Liem A, Miller JA,et al. Activation of the Maillard reaction product 5-(hydroxymethyl)furfural to strong mutagens via allylic sulfonation and chlorination. Chemical Research in Toxicology 1994; 7(3): 313-318.
- Qun T, Zhou T, Hao J, et al. Antibacterial activities of anthraquinones: structure-activity relationships and action mechanisms. RSC Medicinal Chemistry 2023; 14(8): 1446-1471.
- Santander M, Chica V, Correa HAM, et al. Unravelling Cocoa Drying Technology: A Comprehensive Review of the Influence on Flavor Formation and Quality. Foods 2025; 14(5): 721.
- Gill P, Moghadam TT, Ranjbar B. Differential scanning calorimetry techniques: applications in biology and nanoscience. Journal of Biomolecular Techniques 2010; 21(4): 167-193.
- Fan XY, Zhou L, Xing YC, et al. A comprehensive investigation on the chemical changes of traditional Chinese medicine with classic processing technology: Polygonum multiflorum under nine cycles of steaming and sunning as a case study. Analytical and Bioanalytical Chemistry 2024; 416(7): 1733-1744.
- Liu ZL, Chao ZM, Liu YY, et al. Maillard reaction involved in the steaming process of the root of Polygonum multiflorum. Planta Medica 2009; 75(1): 84-88.
- Wang D, Wang XH, Yu X, et al. Pharmacokinetics of anthraquinones from medicinal plants. Frontiers in Pharmacology 2021; 12: 638993.
- Li RL, Gao F, Yan ST, et al. Effects of different processed products of Polygonum multiflorum on the liver. Evidence-Based Complementary and Alternative Medicine 2020; 2020: 5235271.
- Wu X, Chen X, Huang Q, et al. Toxicity of raw and processed roots of Polygonum multiflorum. Fitoterapia 2012; 83(3): 469-475.
- Wang YP, Wang YY. Analysis of the development course of traditional Chinese medicine standardization and recommendations on future work. Guidelines and Standards of Chinese Medicine 2023; 1(1): 1-8.
- Fernandes JM, Cunha, LM, Azevedo, EP, et al. Kalanchoe laciniata and Bryophyllum pinnatum: an updated review about ethnopharmacology, phytochemistry, pharmacology and toxicology. Revista Brasileira de Farmacognosia 2019; 29(4): 529-558.
- Lin LF, Li H, Lin HM, et al. A new perspective on liver injury by traditional Chinese herbs such as Polygonum multiflorum: the geographical area of harvest as an important contributory factor. Frontiers in Pharmacology 2017; 8: 349.
- Xu YD, Liu XJ, Gao YY, et al. Metabolomic analysis revealed the edible and extended-application potential of specific Polygonum multiflorum tissues. Heliyon 2024; 10(4): e25990.
- Zhou LQ, Liu TQ, Yan T, et al. 'Nine Steaming Nine Sun-drying' processing enhanced properties of Polygonatum kingianum against inflammation, oxidative stress and hyperglycemia. Journal of the Science of Food and Agriculture 2024; 104(5): 3123-3138.
- Li HY, Yang JB, Li WF, et al. In vivo hepatotoxicity screening of different extracts, components, and constituents of Polygoni Multiflori Thunb. in zebrafish (Danio rerio) larvae. Biomedicine & Pharmacotherapy 2020; 131: 110524.
- Chen HF, Chen YH, Liu CH, et al. Integrated chemometric fingerprints of antioxidant activities and HPLC–DAD–CL for assessing the quality of the processed roots of Polygonum multiflorum Thunb.(Heshouwu). Chinese Medicine 2016; 11: 18.
- He SB, Zhang X, Lu S, et al. A computational toxicology approach to screen the hepatotoxic ingredients in traditional chinese medicines: Polygonum multiflorum thunb as a case study. Biomolecules 2019; 9(10): 577.
- Peng XY, Yu XY, Lu LZ, et al. Application of handheld near infrared spectrometer in quality control of traditional Chinese medicine: Rapid screening and quantitative analysis of Lonicerae Japonicae Flos adulteration. Spectrochimica Acta. Part A, Molecular and Biomolecular Spectroscopy 2025; 326: 125215.
- Jain SD, Wang Y, Chen X et al. WHO guidelines for quality control of herbal medicines: From cultivation to consumption. International Journal of Pharmaceufical and Chemical Analysis 2024;11(3):212-25.
- Zhang YH, Yang XW, Jia ZX, et al. Proteomics Unravels Emodin Causes Liver Oxidative Damage Elicited by Mitochondrial Dysfunction. Frontiers in Pharmacology 2020; 11: 416.
- Wang X, Ding ZF, Ma KQ, et al. Cysteine-Based Protein Covalent Binding and Hepatotoxicity Induced by Emodin. Chemical Research in Toxicology 2022; 35(2): 293-302.
- Wang YP, Zhao MC, Li B, et al. Advances in the mechanism of emodin-induced hepatotoxicity. Heliyon 2024; 10(13): e33631.
- Gong LP, Shen XH, Huang NN, et al. Research progress on hepatotoxicity mechanism of polygonum multiflorum and its main components. Toxicon 2024; 248: 108040.
- Li YX, Gong XH, Liu MC, et al. Investigation of Liver Injury of Polygonum multiflorum Thunb. in Rats by Metabolomics and Traditional Approaches. Frontiers in Pharmacology 2017; 8: 791.
- Rao T, Liu YT, Zeng XC, et al. The hepatotoxicity of Polygonum multiflorum: The emerging role of the immune-mediated liver injury. Acta Pharmacologica Sinica 2021; 42(1): 27-35.
- Lin EY, Chagnaadorj A, Huang SJ, et al. Hepatoprotective Activity of the Ethanolic Extract of Polygonum multiflorum Thunb. against Oxidative Stress-Induced Liver Injury. Evidence-Based Complementary and Alternative Medicine 2018; 2018: 4130307.
- Teka T, Wang L, Gao J, et al. Polygonum multiflorum: Recent updates on newly isolated compounds, potential hepatotoxic compounds and their mechanisms. Journal of Ethnopharmacology 2021; 271: 113864.
- Woo SM, Davis WD, Aggarwal S, et al. Herbal and dietary supplement induced liver injury: Highlights from the recent literature. World Journal of Hepatology 2021; 13(9): 1019-1041.
- Delgadillo DA, Burch JE, Kim LJ, et al. High-Throughput Identification of Crystalline Natural Products from Crude Extracts Enabled by Microarray Technology and microED. ACS Central Science 2024; 10(1): 176-183.
- Saied EM, El-Maradny YA, Osman AA, et al. A Comprehensive Review about the Molecular Structure of Severe Acute Respiratory Syndrome Coronavirus 2 (SARS-CoV-2): Insights into Natural Products against COVID-19. Pharmaceutics 2021; 13(11): 1759.
- Liu ZL, Chao ZM, Liu YY, et al. Maillard reaction involved in the steaming process of the root of Polygonum multiflorum. Planta Medica 2009; 75(1): 84-88.
- Chen B, Liu S, Xia H, et al. Computer-Aided Drug Design in Research on Chinese Materia Medica: Methods, Applications, Advantages, and Challenges. Pharmaceutics 2025; 17(3): 315.
- Xiao F, Wang H, Guo LP, et al. Application of Blockchain Sharding Technology in Chinese Medicine Traceability System. Computers, Materials and Continua2023; 76(1): 35-48.
- Yan X, Yuan Y, Liao WY et al. Blockchain-based Traditional Chinese Medicine Traceability Model. International Journal of Applied Science 2025; 8(1): 98.
- Lin LF, Li H, Lin HM, et al. Application of iTRAQ-Based Quantitative Proteomics Approach to Identify Deregulated Proteins Associated with Liver Toxicity Induced by Polygonum Multiflorum in Rats. Cellular Physiology and Biochemistry 2017; 43(5): 2102-2116.
- Pang J, Bai Z, Zhao Y et al. Compatibility attenuated detoxification study on radix polygoni multiflori-caused hepatic sinus endothelial cell injury based on high content analysis. Modern Chinese Medicine 2015; 17(4): 331-334.