Main Text
1 Introduction
With the ongoing shift in Traditional Chinese Medicine (TCM) from qualitative to quantitative research [1], the properties of some active ingredients in Chinese herbs are being precisely elucidated. One such compound is formononetin (FMN). FMN, also known as 7-hydroxy, 4'-methoxyisoflavone or biochanin B, is a kind of isoflavone compound extracted from Chinese medicine such as Astragalus membranaceus, Radix Puerariae, Trifolium pratense L., and Caragana jubata (Pall.) Poir., which shares a similar chemical structure with diethylstilbestrol. FMN, a natural phytoestrogen, possessing anti-tumor [2-4], anti-oxidant [5,6], anti-inflammatory [7-9] immunomodulatory [10-12], and estrogen-like properties [13,14].
Organ injuries are common concerns globally, and every year, millions of patients suffer from pain and damage due to organ injuries to different degrees. Various acute injuries, chronic diseases and natural aging are universal inducers of organ injuries, and thus tissue repair and regeneration after organ injury have constantly received attention. Due to the involvement of free radical formation and inflammatory response in the occurrence of various diseases in the human body, the antioxidant activity and anti-inflammatory effects of FMN have been widely studied by scholars domestically and internationally [15]. However, the protective properties of FMN against organ damage and the mechanism by which it promotes repair are not yet fully understood. To address this knowledge gap, this review systematically examines FMN's therapeutic potential across multiple organ systems including the hematopoietic/immune, cardiovascular, nervous, digestive, respiratory, urogenital, and skeletal systems, aiming to provide a comprehensive foundation for future research into FMN's clinical applications for organ protection and regeneration.
2 Hematopoietic immune system
Bone marrow is the main hematopoietic organ of mammals and also one of the central immune organs of the human body.Bone marrow dysfunction not only severely impairs hematopoiesis but also leads to great deficiency in immune function. Aplastic anemia (AA), an autoimmune disease characterized by bone marrow hematopoietic failure, clinically manifests as anemia, infection, and bleeding. Natural products show great potential in alleviating hematopoietic injury. The existing study revealed that FMN can improve hematopoiesis and bone marrow injury in mice with immunity-mediated bone marrow hematopoietic failure, inhibit inflammatory cytokines expression, such as interferon-γ (IFN-γ), tumor necrosis factor-α (TNF-α) and interleukin-12 (IL-12), and restore CD8+/CD4+ T and T-regulatory (Treg)/T helper 17 (Th17) cell balance, thus improving immunologic derangement. The mechanism may be related to the regulation of proteins in the phosphoinositide 3-kinases (PI3K)/ protein kinase B (Akt) pathway [16].
Blood cells produced by the hematopoietic system are crucial to the immune system. FMN has immunomodulatory effects and can repair immune organs and functions. Mao TT et al. [17] discovered that FMN significantly improves the recovery of small intestinal mucosal immune function in immunosuppressed mice, as evidenced by promoting growth of the thymus and spleen, repairing ultrastructural damage in intestinal villi and mucosal epithelial cells, increasing villus height and the villus/crypt ratio, and maintaining the integrity and continuity of small intestinal mucosal epithelial cells. Retinoic acid receptor-related orphan receptor-α (RORα) and receptor-γ (RORγ) are key regulators of Th17 cell differentiation, participating in the innate immune system and autoimmune diseases. Kojima H et al. [18] confirmed that FMN, as a dual agonist of RORα/γ, mediates Th17 cell function, thereby potentially impacting the immune system. FMN also regulates the activity of mouse peritoneal macrophages (PMs) and Kupffer cells (KCs) via the toll-like receptor 4/nuclear factor-κB (TLR4/NF-κB) pathway, and strengthens the regulation of the immune system function of the body [10]. Besides, FMN can promote lymphocyte proliferation and specific cytokine release, enhance humoral and cellular immunity, boost proliferation of liver intrinsic phagocyte KCs and enhance autoimmune function [11]. Meanwhile, FMN has immune-activating effects on antigen-presenting cells (APCs) [12].
3 Cardiovascular system
Cardiovascular system diseases have relatively high mortality among non-communicable diseases currently and are severe health problems at home and abroad. In the research field of cardiocerebral vascular system, FMN has been proved to exert various pharmacological effects, which can significantly ameliorate atherosclerosis and cardiac fibrosis, reduce blood pressure, and optimize the condition of the cardiovascular and cerebrovascular systems by increasing cardiomyocytes and adjusting various influencing factors.
Atherosclerosis (AS) is a pathological process based on the injury of vascular endothelial cells and characterized by lipid infiltration and vascular wall inflammation. Zhang BH et al. [5] exposed human umbilical vein endothelial cells (HUVECs) to oxidized low-density lipoprotein (ox-LDL) to create an in vitro AS model. The results indicated FMN could protect HUVECs from ox-LDL-induced inflammation, oxidative stress, and apoptosis, and protect vascular endothelial cells via attenuating ox-LDL-mediated peroxisome proliferator-activated receptor gamma (PPARγ) signal inactivation. Moreover, FMN can impact the interaction between Krüppel-like factor 4 (KLF4) and steroid receptor RNA activator 1 (SRA1) to decrease monocyte adhesion in apolipoprotein E dual deficient (ApoE–/–) AS model mice, inhibit the formation of foam cells derived from vascular smooth muscle cells (VSMCs) and macrophages, dampen inflammatory responses and alleviate AS development [19]. Furthermore, FMN can further reduce the damaging effects of inflammation on blood vessels and enhance the body's ability to repair atherosclerotic lesions by influencing macrophage polarization, blocking the JAK/STAT signaling pathway, and increasing the expression of α7nAChR [20].
Myocardial ischemia-reperfusion injury (MIRI) can induce cardiac dysfunction and inflammatory responses, particularly the activation of the NOD-like receptor pyridin domain containing 3 (NLRP3) inflammasome. FMN exerts significant myocardial protective effects. It can downregulate the levels of myocardial injury markers and inflammatory factors in MIRI model rats, reduce myocardial infarct size, improve left ventricular systolic function, inhibit cardiomyocyte apoptosis, and simultaneously suppress the activation of the NLRP3 inflammasome and pyroptosis [21]. Additionally, FMN can alleviate myocardial ischemia-reperfusion injury by reducing MIRI-induced platelet activation and NETs formation through the platelet CD36-mediated ERK5 signaling pathway, without increasing the risk of bleeding [22].
Depression is a common complication of myocardial infarction (MI), and the generation of IL-6 and interleukin-17A (IL-17A) post-MI triggers neuroinflammation, severely impacting the prognosis of MI. The immune system serves as a bridge between depression and MI. FMN ameliorates myocardial cell apoptosis, cardiac dysfunction, and depressive symptoms in post-MI depressed mice by targeting glycogen synthase kinase-3β (GSK-3β) to regulate macrophage/microglial polarization [23]. FMN positively affects energy metabolism and cardiac function in mice with heart failure with preserved ejection fraction (HFpEF). Xu H et al. [24] demonstrated that FMN improves HFpEF-induced left ventricular hypertrophy and diastolic dysfunction, enhance the expression of peroxisome proliferator-activated receptor alpha (PPARα), peroxisome proliferator-activated receptor gamma coactivator-1 (PGC-1), and fatty acid metabolism-related genes, and suppresses the expression of glucose metabolism-related genes, which contributes to improving the ability to utilize fatty acids and enhance mitochondrial function. Reportedly, FMN at different dosages protects against dilated cardiomyopathy-induced heart failure, reduces cardiomyocyte apoptosis, and improves cardiac tissue fibrosis [25]. Mitochondrial dysfunction is a key feature of cardiac fibrosis. Upon mitochondrial dysfunction, the energy supply to myocardial cells is insufficient, and oxidative stress levels are increased, thereby promoting excessive deposition of extracellular matrix in myocardial cells and causing or exacerbating myocardial fibrosis [26]. It has been documented that FMN mitigates mitochondrial dysfunction and reduces β-adrenergic-induced cardiac fibrosis by regulating the expression of aldehyde dehydrogenase 2 (ALDH2), hydroxyacyl-CoA dehydrogenase (HADH), and monoamine oxidase B (MAOB) in cardiomyocytes [27], demonstrating potential cardioprotective effects.
4 Nervous system
Alzheimer's disease (AD) is a global neurodegenerative disorder characterized by impaired memory and cognitive function. Its core pathological mechanisms include hippocampal neuronal apoptosis, mitochondrial dysfunction induced by Aβ42 aggregation, and chronic neuroinflammation leading to Aβ plaque deposition, oxidative stress, and neuronal degeneration [28]. Studies have demonstrated that FMN extracted from Pueraria lobata exerts certain preventive and ameliorative effects on AD. Specifically, FMN prevents neuronal apoptosis by inhibiting the Bcl-2 asociated X potein (Bax)/ B-cell lymphoma/leukemia 2 (Bcl-2) pathway, reducing oxidative stress, and stabilizing mitochondrial membranes [6]. A study by Fu et al. [7] indicated that FMN can significantly alleviate learning and memory impairments in mice induced by a high-fat diet, reverse the excessive phosphorylation of hippocampal Tau protein, and decrease the levels of pro-inflammatory cytokines IL-1β and TNF-α. The underlying mechanism is related to the ability of FMN to inhibit the pro-inflammatory NF-κB and enhance the nuclear factor erythroid 2-related factor 2 (Nrf-2)/ heme oxygenase-1 (HO-1) pathway.
Ischemic stroke is a leading cause of death and adult disability worldwide. Studies have shown that FMN exerts a significant protective effect on neurological deficits following ischemic stroke [29]. FMN can improve the neurobehavioral performance of model rats, increase the number of dendritic spines in neurons, upregulate the expression of βIII-tubulin, growth-associated protein 43 (GAP-43), and other related proteins, elevate the levels of nerve growth factor (NGF) and brain-derived neurotrophic factor (BDNF), activate the PI3K/AKT/ERK signaling pathway, and simultaneously exert anti-apoptotic and anti-inflammatory effects, thereby promoting the regeneration and repair of nerves [30].
FMN exhibits significant neuroprotective effects in both ischemic brain injury and reperfusion injury. During the ischemic phase, Chen et al. [31] demonstrated that FMN effectively suppresses the TLR4/NF-κB signaling pathway, reducing nuclear translocation of NF-κB p65 and consequently downregulating the expression of pro-inflammatory cytokines including IL-1β and TNF-α. Importantly, FMN facilitates the phenotypic shift of microglia from pro-inflammatory M1 to anti-inflammatory M2 polarization. This modulatory effect appears to synergize with its PARP1/PARG-mediated upregulation of Iduna [32], collectively attenuating neuroinflammation-induced neuronal damage.
Regarding reperfusion injury, FMN exerts protective effects through multiple mechanisms: (1) modulating the Bcl-2/Bax balance via activation of the ER-PI3K-Akt pathway to inhibit Caspase-3 activation; (2) alleviating oxidative stress by reducing NO/NOS levels; and (3) preserving mitochondrial membrane potential while improving ultrastructural integrity, thereby inhibiting PARP1/AIF-mediated apoptotic pathways [33-36]. Animal studies have confirmed FMN's capacity to significantly reduce cerebral infarct volume and improve neurological function [33,34]. These findings collectively demonstrate that FMN provides multi-target neuroprotection against cerebral ischemic injury through synergistic anti-inflammatory, antioxidant, and anti-apoptotic mechanisms, though further research is warranted to explore its clinical applications.
Spinal cord injury (SCI) is a severe traumatic disorder that leads to sensory and motor dysfunction. Recent studies have confirmed the critical role of inflammatory processes in mediating tissue damage following SCI, which may contribute to neuronal loss and demyelination. Microglia participate in the process after SCI and the occurrence of neuroinflammation [37]. They can be activated and secrete considerable pro-inflammatory cytokines and chemokines to exacerbate microglial inflammation [8]. Inhibiting the activation of microglia can mitigate spinal cord tissue injury. Research found that FMN can curb the activation of microglia, reduce microglial inflammatory response, and improve spinal cord tissue injury and functional recovery by suppressing the epidermal growth factor receptor (EGFR)/ mitogen-activated protein kinase (MAPK) signaling pathway [38].
5 Digestive system
Digestive system diseases have emerged as a major global public health challenge, representing a primary contributor to the societal disease burden and posing severe threats to human health. Research indicates that dysfunction, loss, and apoptosis of intestinal epithelial cells accelerate the pathogenesis of inflammatory bowel disease (IBD) [39]. Furthermore, excessive intestinal immune activation and elevated levels of inflammatory mediators are closely associated with the onset and progression of IBD [40], whereas gut microbiota dysbiosis is a pivotal factor driving inflammatory and immune dysregulation. A study demonstrated that FMN can partially improve the symptoms and inflammatory factors of dextran sulfate sodium-induced colitis in mice and has a restorative effect on gut microbiota [41]. Wu et al. [42] found that FMN can protect against dextran sulfate sodium-induced acute colitis in mice by inhibiting the activation of the NLRP3 inflammasome pathway. FMN also can reduce oxidative stress, restore gut homeostasis, and strengthen the intestinal barrier function to exert certain therapeutic effects on acute pancreatitis [43]. Research findings indicate that FMN suppresses the Hippo/Yes-associated protein (YAP) signaling pathway, upregulates YAP expression, and attenuates intestinal epithelial cell apoptosis, demonstrating protective effects against 2,4,6-trinitrobenzene sulfonic acid (TNBS)-induced IBD in rat models [44]. Separate investigations reveal that pregnancy-induced gut microbiota dysbiosis triggers heterogeneous nuclear ribonucleoprotein U like 2 (hnRNPUL2) nuclear accumulation and macrophage pyroptosis, ultimately leading to immune dysregulation. Importantly, FMN was shown to mitigate this process by inhibiting hnRNPUL2 nuclear translocation and its subsequent binding to the NLRP3 promoter, thereby suppressing macrophage pyroptosis [9].
The liver serves as a crucial hub that is intricately involved in various physiological processes within the body. FMN plays an important role in regulating lipid metabolism and reducing hepatic steatosis. FMN improves hyperlipidemia and hepatic steatosis in high-fat diet (HFD) mice by promoting transcription factor EB (TFEB) mediated lysosomal biogenesis and lipophagy [45]. FMN reduces serum triglycerides (TG), total cholesterol (TC), and low-density lipoprotein cholesterol (LDL-C) levels in HFD mice, improve autophagy flow, induce hepatic cell lipid autophagy, reduce hepatic cell lipid deposition, and mitigate non-alcoholic fatty liver disease [46]. FMN promotes fatty acid β-oxidation and mediates liver lipid metabolism by activating the silencing regulatory protein 1 (SIRT1)/PPARγ coactivator-1α (PGC-1α)/PPARα pathway, ultimately improving hepatic steatosis in non-alcoholic steatohepatitis (NASH) mice [47].
Hepatic fibrosis is a chronic pathological process characterized by excessive deposition of extracellular matrix (ECM) induced by various pathogenic factors [48,49]. Oxidative stress, as a key pathogenic mechanism, promotes abnormal secretion of α-smooth muscle actin (α-SMA) and collagen by activating hepatic stellate cells (HSCs), thereby accelerating fibrotic progression [50,51]. Studies indicate that targeted regulation of oxidative stress, inhibition of HSC activation, and modulation of ECM degradation represent effective strategies for reversing hepatic fibrosis. The research team led by Xie N [52] discovered that FMN can significantly enhance antioxidant enzyme activity by activating the Nrf2/NOX4 pathway, thereby alleviating oxidative stress-induced HSC activation and excessive ECM deposition. Further investigations revealed that FMN also downregulates H3K27me3 epigenetic modification by inhibiting the EZH2/YAP signaling axis, inducing HSC senescence, thus providing dual regulation of HSC activation and ECM metabolism [53]. These findings provide important theoretical evidence for developing FMN as a multi-target anti-fibrotic drug.
6 Respiratory system
Acute lung injury is a disease characterized by respiratory failure, pulmonary edema, alveolar-capillary membrane barrier disruption, and immune/inflammatory responses. FMN displays protective effects on acute lung injury through enhancing the immune function of rats, and the mechanism may involve regulation of the high-mobility group box 1 (HMGB1)/ receptor for advanced glycation end products (RAGE)/NF-κB signaling pathway to reduce the production and release of inflammatory factors and attenuate the inflammatory response and damage in lung tissue [54]. Cell pyroptosis is a pro-inflammatory form of programmed cell death (PCD). It results from the activation of caspase-1 in inflammasome complexes and intracellular lipopolysaccharid (LPS) recognition. This process is closely related to lung injury and fibrosis [55]. FMN can block the activation and pyroptosis of NLRP3 inflammasome in macrophages by regulating lipid metabolism, reducing inflammation and fibrosis, and restoring lung function [56].
Acute pulmonary embolism (APE) occurs when various emboli block the pulmonary artery, leading to respiratory and pulmonary circulation dysfunction. The blockage of the pulmonary artery by emboli can obstruct blood flow and trigger oxidative stress damage, resulting in pulmonary hypertension, while stimulating the vascular endothelium and inducing inflammatory responses to further exacerbate the disease. In APE rat models, FMN evidently alleviates lung tissue lesions. It increases arterial blood oxygen partial pressure and oxygenation index, and reduces serum inflammatory factor levels. Meanwhile, it elevates the expression levels of SOD, Nrf2, and HO-1 in lung tissue, and decreases MDA and p-NF-κB/NF-κB levels. Its mechanism may involve activating the Nrf2/HO-1 signaling pathway to protect lung tissue in APE rats [57].
Asthma is a chronic respiratory disease. Dexamethasone treatment for asthma can exacerbate epithelial damage, accompanied by reduced proliferation and increased death of airway epithelial cells, while FMN can maintain epithelial integrity through the G protein-coupled estrogen receptor (GPER) and effectively reduce dust mite-induced allergic asthma [58]. Airway inflammation and remodeling are fundamental elements of asthma, which cause histological changes in airway structure, including thickening of the airway basement membrane, smooth muscle hyperplasia, and increased fibrosis, leading to decreased lung function [59]. In the ovalbumin (OVA)-induced mouse asthma model, FMN sodium sulfonate can significantly reduce asthma symptom scores, decrease the number of inflammatory cells in bronchoalveolar lavage fluid, and mitigate pathological changes in lung tissue. In addition, FMN sodium sulfonate can also increase the activity of SOD, reduce the levels of inflammatory mediators (such as TNF-α, IL-1β, IL-6, and MDA) and oxidative stress products. Its mechanism may be related to the inhibition of inflammation, the prevention of oxidative damage, and the regulation of the MAPK signaling pathway [60]. FMN promotes the proliferation and migration of LPS-induced bronchial epithelial cells (16HBE), inhibits apoptosis, repairs the epithelial barrier, reduces the content of inflammatory cytokines [interleukin-4 (IL-4), IL-6, interleukin-10 (IL-10), and IL-17A)] in house dust mite (HDM)-induced mouse asthma, and alleviates airway inflammation. The relevant mechanism involves the suppression of estrogen Receptor 1 (ESR1)/NLRP3/Caspase-1 signaling pathway [61]. Yi L et al. [62] demonstrated that by inhibiting NF-κB and c-jun n-terminal kinase (JNK) signaling transduction and enhancing Nrf2 signaling transduction, FMN dampens the expression of Th2-related inflammatory cytokines [(such as IL-4, interleukin-5 (IL-5), and interleukin-13 (IL-13)] and IgE in OVA-induced asthma models, alleviating OVA-induced airway inflammation and remodeling.
7 Urinary and reproductive systems
FMN is a type of isoflavone phytoestrogen with weak estrogenic activity and bidirectional regulatory effects [63]. Studies have shown that FMN can significantly improve ovarian and uterine weight in mice [64]. At high doses, it promotes endometrial hyperplasia and induces vaginal epithelial keratinization in ovariectomized rats, yet exhibits minimal effects on the human reproductive system [65]. Its protective mechanisms include:Inhibiting the TLR4/MyD88/NF-κB pathway to reduce inflammatory damage in endometrial epithelial cells [66]; Regulating the expression of p27, pSTAT3, and other key molecules to treat endometriosis [67]; Mitigating benign prostatic hyperplasia via estrogen receptor signaling [13].
In terms of renal protection, FMN exerts its effects through multiple mechanisms: Enhancing antioxidant enzymes (SOD, GSH, etc.) to alleviate oxidative stress and metabolic disorders in diabetic nephropathy [68]; Activating the SIRT1/Nrf2 pathway to reduce high glucose-induced renal fibrosis [69]; Modulating the Smad3/ATF3/SLC7A11 signaling axis to suppress tubular epithelial cell fibrosis and apoptosis [70]. Additionally, FMN has been shown to improve acute kidney injury in sepsis [71] and diabetes-related cognitive dysfunction [14].
These findings collectively suggest FMN as a promising multi-target agent for hormone-related disorders and kidney diseases, though further clinical investigations are required to verify its therapeutic efficacy.
8 Skeletal system
FMN demonstrates multifaceted therapeutic potential for skeletal system disorders. In osteoporosis (OP), FMN (10-6 mol/L) significantly promotes the proliferation and differentiation of MC3T3-E1 osteoblasts by activating the Wnt/β-catenin signaling pathway, suggesting its potential to improve bone loss and microstructural damage through enhanced bone formation [72].
For osteoarthritis (OA) treatment, FMN exhibits multi-target effects: it reduces levels of pro-inflammatory factors including TNF-α, IL-1β, and IL-6 by inhibiting the NF-κB signaling pathway, thereby alleviating cartilage inflammation [73]; meanwhile, it regulates the MAPK/NF-κB pathway to downregulate the expression of catabolic proteins such as MMP13, p-ERK, p-JNK, and p-p38 while promoting type II collagen synthesis to protect cartilage matrix [74]. Furthermore, FMN specifically inhibits Th17 cell differentiation and IL-26 secretion, blocking the chronic inflammatory process mediated by Th17 cells [75-77]. These findings indicate that FMN exerts multiple pharmacological effects including "bone formation promotion, anti-inflammation, and cartilage protection" in skeletal system diseases through coordinated regulation of the Wnt/β-catenin, NF-κB and MAPK signaling networks, demonstrating significant clinical application value.
9 Other organs
FMN also has antibacterial, wound healing-promoting, blood glucose levels-controlling, and depression- and anxiety-alleviating properties, and shows positive effects on damaged skin, metabolic injuries, and emotional regulation. In antimicrobial applications, FMN effectively inhibits Streptococcus suis infection both in vivo and in vitro by targeting hemolysin [78]. Its anti-inflammatory mechanisms include: alleviating atopic dermatitis through GPER-mediated upregulation of TNFAIP3 expression [79]; and suppressing LPS-induced NF-κB signaling activation, reducing pro-inflammatory cytokines (e.g., TNF-α, IL-1β), while enhancing tight junction protein expression to protect blood-milk barrier integrity, thereby mitigating mastitis.
Regarding metabolic regulation, FMN exhibits hypoglycemic effects in alloxan-induced type 1 diabetic mice by inhibiting pancreatic β-cell apoptosis, promoting regeneration and insulin secretion, as well as enhancing hepatic glycogen synthesis and glycolysis [80]. Furthermore, FMN modulates neuroinflammation in chronic unpredictable mild stress (CUMS)-induced depressive rats by downregulating the TLR4/MyD88/NF-κB pathway, ameliorating cognitive dysfunction and depressive behaviors [81].
In reproductive health, FMN counteracts oxidative stress in gestational diabetic rats by elevating GSH, SOD, and total antioxidant capacity (TAC) levels, restoring placental ultrastructure and improving pregnancy outcomes [82]. FMN also demonstrates therapeutic potential for allergic rhinitis by inhibiting IL-13-induced airway inflammation and mucus hypersecretion via the SIRT1/Nrf2 pathway [83]. Notably, FMN accelerates endothelial repair and wound healing through ERK1/2 and p38 MAPK-mediated EGR-1 overexpression [84], highlighting its applicability in tissue regeneration.
The mechanisms by which FMN protects against organ injury and promotes tissue regeneration/repair are illustrated in Figure 1 and Table 1.
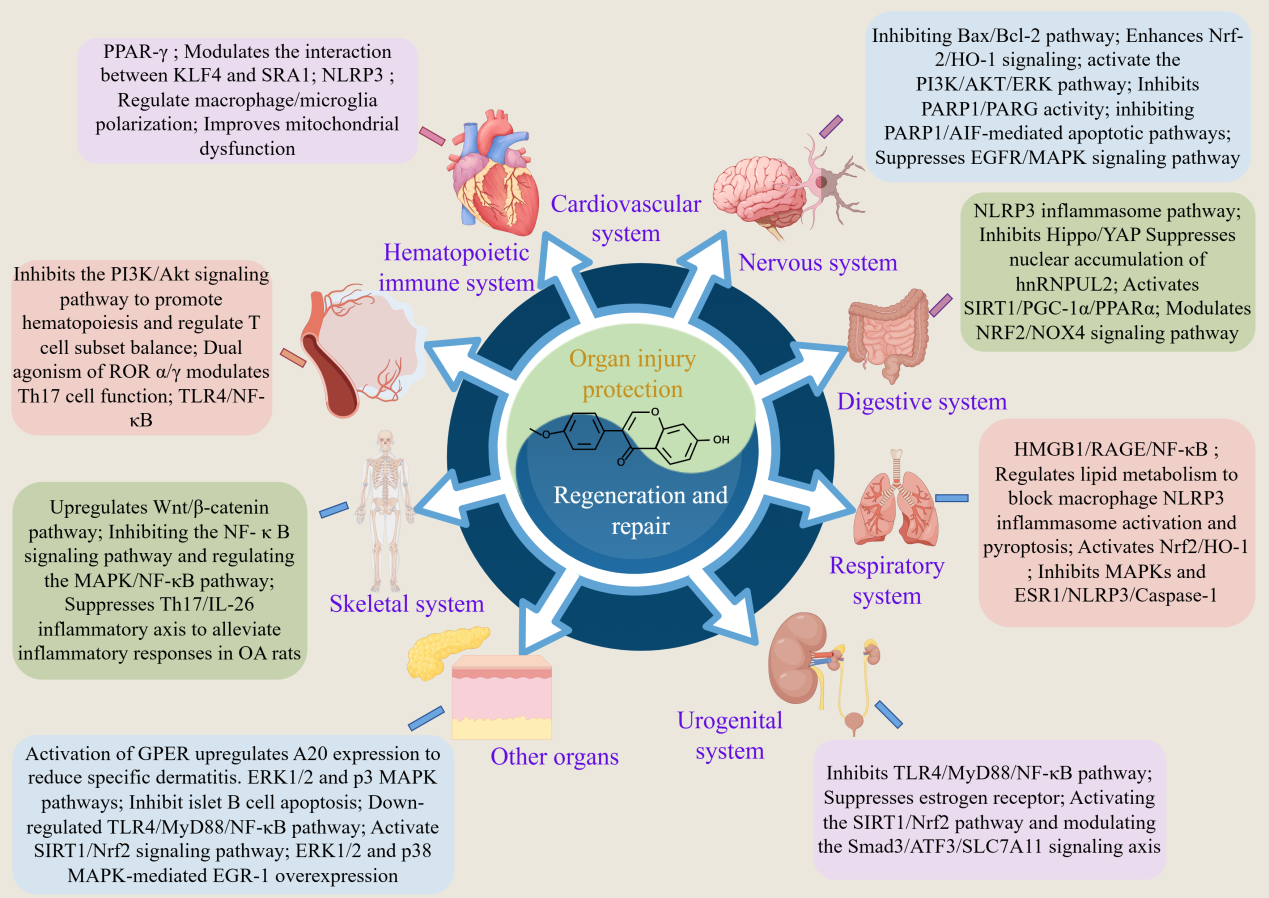
Figure 1 The mechanism of FMN protecting organ injuries and promoting regeneration and repair.
Table 1 The mechanism of FMN protecting organ injuries and promoting regeneration and repair.
| System | Injuried organ/ Diseases | Protective effects/Mechanism | References |
|---|---|---|---|
| Hematopoietic immune system | Aplastic anemia | Reverse Treg/Th17 imbalance through regulating the PI3K/Akt signaling pathway | [16] |
| Immunosuppression | Improve and promote the recovery of intestinal mucosal immune function | [17] | |
| Immunity dysfunction | Modulate immunity via RORα/γ dual agonism regulating Th17 cells | [18] | |
| Cardiovascular system | Atherosclerosis | Protect endothelial function via PPAR-γ activation against ox-LDL | [5] |
| Regulate the KLF4-SRA interaction, which encompasses reducing foam cell formation, inhibiting monocyte adhesion and inflammation, and stabilizing plaques | [19] | ||
| Regulate macrophage polarization, inhibit JAK/STAT signaling, upregulate α7nAChR, reduce inflammation, and lower lipid levels | [20] | ||
| Myocardial ischemia-reperfusion injury | Mitigate myocardial ischemia/reperfusion injury through ROS-TXNIP-NLRP3 pathway inhibition | [21] | |
| Mitigate myocardial ischemia/reperfusion injury through platelet CD36-mediated ERK5 signaling | [22] | ||
| Model of myocardial infarction combin | Modulate macrophage/glial cell polarization through GSK-3β targeting | [23] | |
| Heart failure with preserved ejection fraction | Activate PPARα/PGC-1 pathway and regulate energy metabolism | [24] | |
| Heart failure | Modulate HSP90/AKT pathway by inhibiting HSP90, promoting AKT phosphorylation, and reducing CASP3 | [25] | |
| Cardiac fibrosis | Improve mitochondrial dysfunction by regulating the expressions of cardiomyocyte ALDH2, HADH, and MAOB | [27] | |
| Nervous system | Alzheimer's disease | Prevent neuronal apoptosis via Bax/Bcl-2 pathway inhibition, oxidative stress reduction, and mitochondrial membrane stabilization | [6] |
| Cognitive disorde | Modulate metabolism, suppress neuroinflammation and Tau hyperphosphorylation through PGC-1α pathway modulation, NF-κB inhibition, and Nrf-2/HO-1 activation | [7] | |
| Ischemic stroke | Enhanced neurobehavior, increased dendritic spine density, upregulated neurotrophic factor expression, activated PI3K/AKT/ERK signaling, and exerted anti-apoptotic/anti-inflammatory effects | [30] | |
| Inhibit PARP1/PARG activity, promote the expression of the neuroprotective factor Iduna, and inhibit NF-κB transcription as well as the secretion of pro-inflammatory factors | [32] | ||
| Alleviate neuroinflammation by inhibiting the TLR4/NF-κB pathway, reducing nuclear translocation of NF-κB p65, downregulating pro-inflammatory factors, and promoting microglial polarization from the M1 to M2 phenotype | [31] | ||
| Cerebral ischemia/reperfusion injury | Anti-oxidative stress, anti-inflammation, inhibition of neuronal apoptosis, and improvement of mitochondrial ultrastructural damage | [33-36] | |
| Spinal cord injury | Curb microglial activation and reduce microglial inflammatory responses by suppressing the EGFR/MAPK signaling pathway | [38] | |
| Digestive system | Inflammatory bowel disease | Inhibit activation of the NLRP3 inflammasome pathway, block the Hippo/YAP signaling pathway, promote YAP expression, and reduce intestinal epithelial cell apoptosis | [41,42,44] |
| Disorder of intestinal flora | Dampen macrophage pyroptosis by inhibiting nuclear accumulation of hnRNPUL2 and its subsequent binding to the NLRP3 promoter | [9] | |
| Fatty liver | Activate TFEB-mediated lysosomal biogenesis, improve hepatocyte autophagy function, activate the SIRT1/PGC-1α/PPARα pathway, and promote hepatic fatty acid β-oxidation | [45,47] | |
| Hepatic fibrosis | Enhance the activity of antioxidant enzymes, weaken inflammatory responses and excessive expression of extracellular matrix, and regulate the Nrf2/NOX4 signaling pathway to inhibit oxidative stress | [52] | |
| Suppress the EZH2/YAP axis enhancer, downregulate EZH2 and its catalytic product H3K27me3, induce HSC senescence, and thereby inhibit HSC activation and extracellular matrix accumulation | [53] | ||
| Respiratory system | Acute lung injury | Regulate the HMGB1/RAGE/NF-κB signaling pathway, reduce the degree of inflammation and damage in lung tissue, and improve immune function | [54] |
| Pulmonary fibrosis | Block macrophage NLRP3 inflammasome activation and pyroptosis via mediating lipid metabolism, thereby reducing inflammatory responses and alleviating fibrosis | [56] | |
| Acute pulmonary embolism | Activate the Nrf2/HO-1 signaling pathway to inhibit oxidative stress and reduce inflammatory responses | [57] | |
| Asthma | Inhibit the MAPKS and ESR1/NLRP3/Caspase-1 signaling pathways to attenuate inflammatory responses | [60,61] | |
| Urogenital system | Pelvic inflammatory disease | Inhibit activation of the TLR4/MyD88/NF-κB pathway and reduce levels of TNF-α, IL-1β, and IL-6 in endometrial epithelial cells | [66] |
| Endometriosis | Regulate the expressions of key molecules such as p27, pSTAT3, and progesterone receptors | [67] | |
| Benign prostatic hyperplasia | Act on estrogen receptors such as ER-α and ER-β, and regulate cell cycle-related genes including CDK1, cyclin A2, CDK2, and cyclin B1 | [13] | |
| Renal fibrosis | Activate the Nrf2/ARE signaling pathway via Sirt1 and suppress the Smad3/ATF3/SLC7A11 signaling pathway | [69,70] | |
| Skeletal system | Osteoporosis | Activation and upregulation of the Wnt/β-catenin pathway | [72] |
| Osteoarthritis | Downregulate Th17 cell differentiation in OA rats and inhibit the Th17/IL-26 inflammatory axis to alleviate inflammation | [77] |
10 Conclusion
FMN, as the primary active component in Astragalus membranaceus, Radix Puerariae, and other traditional Chinese medicine materials (TCM), has been confirmed in substantial studies to exert protective effects on multiple organ injuries in the hematopoietic immune system, cardiovascular system, and nervous system. The anti-inflammatory, anti-oxidative and immune-regulatory properties of FMN can promote regeneration and repair of damaged tissues, and FMN is a potent active ingredient of TCM with great potential for clinical application and development. However, these experiments still remain at the experimental research stage, and the positive effects of FMN on protecting organ injury and promoting regeneration and repair need to be further validated in clinical studies. Meanwhile, FMN is a weakly polar molecule that is almost insoluble in water. Oral administration has unsatisfactory effects, while continuous high-concentration administration often leads to serious adverse reactions. Therefore, new methods such as changing the structure of FMN, effectively improving water solubility, and enhancing bioavailability still require further studies, and further exploration is needed to improve clinical application value.
Back Matter
Acknowledgements
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Substantial contributions to conception and design: Y.M. Data acquisition, data analysis and interpretation: Y.M. Drafting the article or critically revising it for important intellectual content: D.S. Final approval of the version to be published: All authors. Agreement to be accountable for all aspects of the work in ensuring that questions related to the accuracy or integrity of the work are appropriately investigated and resolved: All authors.
Ethics Approval and Consent to Participate
No ethical approval was required for this review.
Funding
This study was supported by Research Project of Zhejiang Provincial Administration of Traditional Chinese Medicine (No. 2025ZR194), Ningbo City Veteran Herbal Medicine Experts Inheritance Studio Construction Project, and Zhejiang Provincial Natural Science Foundation of China (No. LQN25H090018).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- Bai ZG, Zhu YM, Shi NN. Evidence-based research of the indicator system for evaluating the application effect of TCM standards. Guidelines and Standards in Chinese Medicine 2024; 2(4): 131-140.
- Xin M, Wang Y, Ren QY, et al. Formononetin and metformin act synergistically to inhibit growth of MCF-7 breast cancer cells in vitro. Biomedicine & Pharmacotherapy 2019; 109: 2084-2089.
- Yu XY, Gao F, Li W, et al. Formononetin inhibits tumor growth by suppression of EGFR-Akt-Mcl-1 axis in non-small cell lung cancer. Journal of Experimental & Clinical Cancer Research 2020; 39(1): 1-17.
- Wang WS, Zhao CS. Formononetin exhibits anticancer activity in gastric carcinoma cell and regulating miR‐542‐5p. The Kaohsiung Journal of Medical Sciences 2021; 37(3): 215-225.
- Zhang BH, Hao ZW, Zhou WL, et al. Formononetin protects against ox-LDL-induced endothelial dysfunction by activating PPARγ signaling based on network pharmacology and experimental validation. Bioengineered 2021; 12(1): 4887-4898.
- Liu QY, Zou YF, Li Y. Mechanism investigation of Pueraria-extracted formononetin on Alzheimer's Disease. Journal of Wuhan Polytechnic University 2024; 43(04): 23-32+48.
- Fu XX, Qin TT, Yu JY, et al. Formononetin ameliorates cognitive disorder via PGC-1α pathway in neuroinflammation conditions in high-fat diet-induced mice. CNS & Neurological Disorders-Drug Targets 2019; 18(7): 566-577.
- Gao ZS, Zhang CJ, Xia N, et al. Corrigendum to ‘Berberine-loaded M2 macrophage-derived exosomes for spinal cord injury therapy’ [Acta Biomaterialia 126 (2021) 211–223]. Acta Biomaterialia 2022; 140: 745-746.
- Chen X, Wu R, Li L, et al. Pregnancy-induced changes to the gut microbiota drive macrophage pyroptosis and exacerbate septic inflammation. Immunity 2023; 56(2): 336-352. e9.
- Liu S. TLR4/NF-ĸB-mediated formononetin on the immune regulation of mouse peritoneal macrophages and KCs cells. Gansu Agricultural University 2021.
- Zhang YP, Deng K, Jia N, et al. The Effect of Formononetin on the Immune Function in Immunosuppressed Mice. Chinese Animal Husbandry and Veterinary Medicine 2020; 47(03): 922-930.
- Lu R, Wang S, Wang B. Immune activation effects of four traditional Chinese medicine monomers on antigen-presenting cells. Journal of Precision Medicine 2022; 37(04): 311-315+321.
- Wang XH, Wang L, Xiao LJ, et al. Formononetin inhibits benign prostatic hyperplasia through estrogen receptors. Journal of Chinese Pharmaceutical Sciences 2024; 33(03): 216-229.
- Wang JC, Wang L, Qin AP, et al. Improvement of formononetin on cognitive dysfunction in diabetic mice. Chinese Journal of Clinical Rational Drug Use 2022; 09: 1-5+9.
- Li SQ, Wu GX, Liu YL, et al. Research Progress on Pharmacological Effects and Mechanisms of Formononetin. Modern Chinese Medicine 2024; 26(09): 1608-1617.
- Lan HX, Qiu W, Wu J, et al. Formononetin reverses Treg/Th17 imbalance in immune-mediated bone marrow failure mice by regulating the PI3K/Akt signaling pathway. Chinese Medicine 2024; 19(1): 55.
- Mao TT, Bai H, Jia N. Effects of Formononetin on Mucosa Immunity Function in Small Intestinal of Immunosuppressive Mice. Chinese Journal of Animal Nutrition 2022; 34(04): 2712-2720.
- Kojima H, Takeda Y, Muromoto R, et al. Isoflavones enhance interleukin-17 gene expression via retinoic acid receptor-related orphan receptors α and γ. Toxicology 2015; 329: 32-39.
- Ma CR, Xia RL, Yang S, et al. Formononetin attenuates atherosclerosis via regulating interaction between KLF4 and SRA in apoE-/- mice. Theranostics 2020; 10(3): 1090-1106.
- He Y, Cai YD, Wei DL, et al. Elucidating the mechanisms of formononetin in modulating atherosclerotic plaque formation in ApoE-/- mice. BMC Cardiovascular Disorders 2024; 24(1): 121.
- Wang DS, Yan LY, Yang DZ, et al. Formononetin ameliorates myocardial ischemia/reperfusion injury in rats by suppressing the ROS-TXNIP-NLRP3 pathway. Biochemical and Biophysical Research Communications 2020; 525(3): 759-766.
- Tang S, Ye JX, Li RY, et al. Formononetin attenuates myocardial ischemia/reperfusion injury by regulating neutrophil extracellular traps formation and platelet activation via platelet CD36. Phytomedicine 2025; 141: 156736.
- Yang Y, Huang T, Zhang HL, et al. Formononetin improves cardiac function and depressive behaviours in myocardial infarction with depression by targeting GSK-3β to regulate macrophage/microglial polarization. Phytomedicine 2023; 109: 154602.
- Xu H, Zhang XQ, Huang LM, et al. Formononetin improves heart failure with preserved ejection fraction in mice by activating the PPARα/PGC-1 pathway. Traditional Medicine Research 2024; 9(4): 20.
- Qi YY, Xue SY, Chen WJ, et al. Formononetin regulates dilated cardiomyopathy-mediated heart failure in rats via HSP90/Akt cardiomyocyte apoptosis and mechanism. Journal of Xi'an Jiaotong University (Medical Sciences) 2023; 44(05): 794-801.
- Li XY, Zhang W, Cao QT, et al. Mitochondrial dysfunction in fibrotic diseases. Cell Death Discovery 2020; 6(1): 80.
- Qian L, Xu H, Yuan RQ, et al. Formononetin ameliorates isoproterenol induced cardiac fibrosis through improving mitochondrial dysfunction. Biomedicine & Pharmacotherapy 2024; 170: 116000.
- Arjmand S, Ilaghi M, Sisakht AK, et al. Regulation of mitochondrial dysfunction by estrogens and estrogen receptors in Alzheimer's disease: A focused review. Basic Clin Pharmacol Toxicol 2024; 135(2): 115-132.
- Ma XY, Wang JJ. Formononetin: a pathway to protect neurons. Frontiers in Integrative Neuroscience 2022; 16: 908378.
- Wu QL, Cheng YQ, Liu AJ, et al. Formononetin recovered injured nerve functions by enhancing synaptic plasticity in ischemic stroke rats. Biochemical and Biophysical Research Communications 2020; 525(1): 67-72.
- Chen J, Cai YD, Wei DL, et al. Formononetin inhibits neuroinflammation in BV2 microglia induced by glucose and oxygen deprivation reperfusion through TLR4/NF-κB signaling pathway. Brain Research 2024; 1845: 149218.
- Wei DL, Wang M, Wang WX, et al. Protective effects of formononetin against oxygen-glucose deprivation reperfusion injury in BV2 microglia and associated mechanism. Journal of Chongqing Medical University 2025; 50(02): 244-249.
- Gu Y, Chen X, Fu SP, et al. Astragali Radix isoflavones synergistically alleviate cerebral ischemia and reperfusion injury via activating estrogen receptor-PI3K-Akt signaling pathway. Frontiers in Pharmacology 2021; 12: 533028.
- Zou XR, Xu L. Protective Effects and Mechanism Study of Formononetin on Cerebral Ischemia-reperfusion Injury in Rats. Chinese Journal of Modern Applied Pharmacy 2018; 35(06): 855-858.
- Yu L, Wang M, Wang WX, et al. The effect of formononetin on the neuron cell damage of oxygen-glucose deprivation/reoxygenation was studied based on the PARP1 signaling pathway. Acta Universitatis Medicinalis Anhui 2024; 59(02): 207-211.
- Li YN, Li SB, Bai Y, et al. Sodium formononetin-3'-sulphonate alleviates cerebral ischemia-reperfusion injury via regulating mitochondrial apoptosis pathway. Chinese Herbal Medicine 2024; 55(11): 3759-3767.
- Lin ZH, Wang SY, Chen LL, et al. Methylene Blue Mitigates Acute Neuroinflammation after Spinal Cord Injury through Inhibiting NLRP3 Inflammasome Activation in Microglia. Frontiers in Cellular Neuroscience 2017; 11: 391.
- Fu HP, Li MD, Huan YQ, et al. Formononetin inhibits microglial inflammatory response and contributes to spinal cord injury repair by targeting the EGFR/MAPK pathway. Immunological Investigations 2023; 52(4): 399-414.
- Bruner LP, White AM, Proksell S. Inflammatory bowel disease. Primary Care 2023; 50(3): 411-427.
- Zhang J, Cen L, Zhang XF, et al. MPST deficiency promotes intestinal epithelial cell apoptosis and aggravates inflammatory bowel disease via AKT. Redox Biology 2022; 56: 102469.
- Li T, Zou QP, Mao ZW, et al. Therapeutic effect of flavonoids from Desmodium triflorum on an ulcerative colitis mouse model and the influence on intestinal flora. Chinese Journal of Comparative Medicine 2022; 32(04): 29-38.
- Wu DC, Wu KY, Zhu QT, et al. Formononetin administration ameliorates dextran sulfate sodium-induced acute colitis by inhibiting NLRP3 inflammasome signaling pathway. Mediators of Inflammation 2018; 2018: 3048532.
- Yang J, Sha XW, Wu D, et al. Formononetin alleviates acute pancreatitis by reducing oxidative stress and modulating intestinal barrier. Chinese Medicine 2023; 18(1): 78.
- Xie D, Liu YY, Li ZX, et al. Effects of formononetin on the apoptosis of intestinal epithelial cells in rats with inflammatory bowel disease by regulating the Hippo/YAP signaling pathway. China Pharmacy 2024; 35(13): 1564-1569.
- Wang Y, Zhao HL, Li X, et al. Formononetin alleviates hepatic steatosis by facilitating TFEB-mediated lysosome biogenesis and lipophagy. The Journal of Nutritional Biochemistry 2019; 73: 108214.
- Li P, Peng L, Wang Y, et al. The application of formononetin in the treatment of non-alcoholic fatty liver disease: CN110090209A [P]. 2019-08-06.
- Liao JB, Xue XH, Wang N, et al. Formononetin promotes fatty acid β-oxidation to treat non-alcoholic steatohepatitis through SIRT1/PGC-1α/PPARα pathway. Phytomedicine 2024; 124: 155285.
- Altamirano-Barrera A, Barranco-Fragoso B, Méndez-Sánchez N. Management strategies for liver fibrosis. Annals of Hepatology 2017; 16(1): 48-56.
- Aydın MM, Akçalı KC. Liver fibrosis. The Turkish Journal of Gastroenterology 2018; 29(1): 14-21.
- Ramos-Tovar E, Muriel P. Molecular Mechanisms That Link Oxidative Stress, Inflammation, and Fibrosis in the Liver. Antioxidants (Basel) 2020; 9(12): 1279.
- Wang T, Zhou X, Kuang G, et al. Paeoniflorin modulates oxidative stress, inflammation and hepatic stellate cells activation to alleviate CCl4-induced hepatic fibrosis by upregulation of heme oxygenase-1 in mice. Journal of Pharmacy and Pharmacology 2021; 73(3): 338-346.
- Xie N, Ma R, Wang L, et al. Effect of formononetin on glucose oxidase-induced oxidative stress in rat hepatic stellate cells. Chinese Journal of Tissue Engineering Research 2023; 27(33): 5309-5313.
- Yuan Z, Meng JH, Shen XG, et al. Formononetin Mitigates Liver Fibrosis via Promoting Hepatic Stellate Cell Senescence and Inhibiting EZH2/YAP Axis. Journal of Agricultural and Food Chemistry 2024; 72(41): 22606-22620.
- Li H, Li LL. Effects and mechanism of formononetin on acute lung injury and immune function in septic rats. Anatomy Research 2024; 46(03): 236-241.
- Shi ZJ, Yuan SY, Shi LL, et al. Programmed cell death in spinal cord injury pathogenesis and therapy. Cell Proliferation 2021; 54(3): e12992.
- Ouyang BS, Deng LL, Yang FY, et al. Albumin-based formononetin nanomedicines for lung injury and fibrosis therapy via blocking macrophage pyroptosis. Materials Today Bio 2023; 20: 100643.
- Zhao BT, Mao HY, Yao CX, et al. Influence of formononetin on lung tissue damage in rats with acute pulmonary embolism by regulating Nrf2/HO-1 signaling pathway. Journal of North Sichuan Medical College 2023; 38(12): 1592-1597.
- Yuan WY, Li LQ, Chen YY, et al. Frontline Science: Two flavonoid compounds attenuate allergic asthma by regulating epithelial barrier via G protein-coupled estrogen receptor: Probing a possible target for allergic inflammation. Journal of Leucocyte Biology 2020; 108(1): 59-71.
- Lambrecht BN, Hammad H. The immunology of asthma. Nature Immunology 2015; 16: 45-56.
- Chen LC, Yang F. Study on the effect and mechanism of sodium formononetin-3'-sulfonate on OVA-induced asthma mice. Journal of Tianjin University of Traditional Chinese Medicine 2024; 43(05): 407-412.
- Zhang L, Wu Q, Huang YY, et al. Formononetin ameliorates airway inflammation by suppressing ESR1/NLRP3/Caspase-1 signaling in asthma. Biomedicine & Pharmacotherapy 2023; 168: 115799.
- Yi L, Cui J, Wang WQ, et al. Formononetin attenuates airway inflammation and oxidative stress in murine allergic asthma. Frontiers in Pharmacology 2020; 11: 533841.
- Wang J, Sun Y, Chen L, et al. Advances in modern research of formononetin. Journal of Shanxi University of Chinese Medicine 2017; 18(05): 74-76+79.
- Yatkin E, Daglioglu S. Evaluation of the estrogenic effects of dietary perinatal Trifolium pratense. Journal of Veterinary Science 2011; 12(2): 121-126.
- Xing DX, Liu ZG, Xue CK, et al. Study on the estrogen-like effects of formononetin and its correlation with blood lipids. Chinese Journal of Gerontology 2009; 29(18): 2340-2342.
- Ji XL, Yang CC, Huang L, et al. Formononetin Ameliorates LPS-Induced Endometrial Epithelial Cell Injury in Rats via TLR4/MyD88/NF-κB Signaling Pathway. Pharmacology and Clinics of Chinese Materia Medica 2024; 40(02): 62-67.
- Park Y, Choo SP, Jung GS, et al. Formononetin Inhibits Progression of Endometriosis via Regulation of p27, pSTAT3, and Progesterone Receptor: In Vitro and In Vivo Studies. Nutrients 2023; 15(13): 3001.
- Jain P, Nayse PG, Patil DJ, et al. The possible antioxidant capabilities of formononetin in guarding against streptozotocin-induced diabetic nephropathy in rats. Future Journal Of Pharmaceutical Sciences 2020; 6(1): 414-422.
- Zhuang K, Jiang XY, Liu RB, et al. Formononetin activates the Nrf2/ARE signaling pathway via sirt1 to improve diabetic renal fibrosis. Frontiers in Pharmacology 2020; 11: 616378.
- Zhu BW, Ni YF, Gong Y, et al. Formononetin ameliorates ferroptosis-associated fibrosis in renal tubular epithelial cells and in mice with chronic kidney disease by suppressing the Smad3/ATF3/SLC7A11 signaling. Life Sciences 2023; 315: 121331.
- Zhou HY, Luo L, Chen YY, et al. Effects of Formononetin on Sepsis Induced Acute Kidney Injury in Rats by Regulating the SIRT1/PGC-1α Pathway. Guiding Journal of Traditional Chinese Medicine and Pharmacy 2022; 01: 6-11.
- Cheng L. A preliminary study on the effect of formononetin on mouse parietal bone cell. Master’s Degree. Zhengzhou University: Zhengzhou, China, 2022.
- Tian FX, Ding P, Zhou LL. Effect of Formononetin on Alleviating Inflammatory Injury of Chondrocytes via NF-κB Signaling Pathway. Herald of Medicine 2024; 43(11): 1728-1732.
- Xu F. Effect of Ononin on IL-1β-induced Chondrocyte Matrix Metabolism and Inflammation by Downregulating the MAPK and NF-κB Pathways. Master's Degree. Guangxi Medical University: Guangxi, China, 2022.
- Zhong W, Feng LH, Tian W, et al. SMURF1 inhibits the Th17 and Th17.1 polarization and improves the Treg/Th17 imbalance in systemic lupus erythematosus through the ubiquitination of RORγt. Molecular Immunology 2023; 157: 186-194.
- Keller LE, Tait Wojno ED, Begum L, et al. T Helper 17–Like Regulatory T Cells in Equine Synovial Fluid Are Associated With Disease Severity of Naturally Occurring Posttraumatic Osteoarthritis. The American Journal of Sports Medicine 2023; 51(4): 1047-1058.
- Bi B, Gou PG, Huang XH. Effects of formononetin on inflammatory response, cartilage damage and T helper cell 17/interleukin-26 inflammatory axis in rats with osteoarthritis. Chinese Journal of Experimental Surgery 2024; 41(1): 87-90.
- Wang GZ, Liu HM, Liu Y, et al. Formononetin alleviates Streptococcus suis infection by targeting suilysin. Microbial Pathogenesis 2020; 147: 104388.
- Yuan WY, Chen YY, Zhou YJ, et al. Formononetin attenuates atopic dermatitis by upregulating A20 expression via activation of G protein-coupled estrogen receptor. Journal of Ethnopharmacology 2021; 266: 113397.
- Qiu GZ, Tian W, Huan M, et al. Formononetin exhibits anti-hyperglycemic activity in alloxan-induced type 1 diabetic mice. Experimental Biology and Medicine 2017; 242(2): 223-230.
- Zhang CH, Hu LY, Xie Y, et al. Formononetin improves cognitive behavior in aging rats with chronic unpredictable mild in hippocampal tissue stress by blocking the NF-κB pathway and inhibiting the release of inflammatory factors. Chinese Journal of Cellular and Molecular Immunology 2023; 39(07): 610-616.
- Tian YJ, Yang X, Wang J, et al. Influence of formononetin on oxidative stress injury in gestational diabetes mellitus rats. Tianjin Medical Journal 2023; 51(07): 734-739.
- Huang JJ, Chen XF, Xie AH. Formononetin ameliorates IL-13-induced inflammation and mucus formation in human nasal epithelial cells by activating the SIRT1/Nrf2 signaling pathway. Medicine Reports 2021; 24(6).
- Cheng HL, Tao CL, Chen WD, et al. Research Progress on the Pharmacological Effects of Isoflavones from Trifolium pratense L. Journal of Anhui University of Chinese Medicine 2011; 04: 76-80.