Main Text
1 Introduction
Metastatic breast cancer is the second leading cause of cancer-related deaths among women, with an estimated 39,620 fatalities in the United States in 2013. Despite ongoing declines in breast cancer incidence and mortality rates, the survival rate for metastatic cases remains low [1-3]. Conventional treatments, including surgery, radiotherapy, and chemotherapy, have shown limited efficacy in eradicating metastatic breast cancer [4-6]. This underscores the urgent need for novel therapeutic strategies.
Angiogenesis, the process of forming new blood vessels from preexisting ones, is a crucial factor in cancer progression. Growing evidence suggests that angiogenesis is not only significant in hematologic malignancies but also plays a vital role in tumor growth, invasion, and metastasis [7]. Although the precise mechanisms of pathological angiogenesis remain unclear, research suggests that an imbalance between angiogenic stimulators and inhibitors drives this process. When endothelial cells receive pro-angiogenic signals, they activate and release enzymes that degrade the extracellular matrix, facilitating cell migration, proliferation, and eventual differentiation into new capillaries. Targeting any of these steps presents a potential avenue for therapeutic intervention in angiogenesis-dependent diseases [8].
Ursolic acid (UA), a pentacyclic triterpene acid found in numerous Chinese medicinal herbs, exhibits a wide range of pharmacological effects, including anti-inflammatory, hepatoprotective, antiviral, antioxidant, cytotoxic, antitumor, anti-angiogenic, and anti-metastatic properties [9-13]. Some studies have highlighted the inhibitory effects of UA in the chorioallantoic membrane (CAM) assay [14], as well as its differential influence on endothelial cell proliferation and angiogenesis [15].
In our previous research, we employed a stage IV breast cancer mouse model (4T1 tumor-bearing BALB/c mice) to assess the anti-metastatic potential of UA [16]. 4T1 cells, originating from a spontaneous mammary tumor in BALB/c mice, exhibit aggressive growth when implanted into the mammary fat pad of syngeneic mice and readily metastasize to the lungs, liver, bones, and brain [17,18]. In this report, laser Doppler imager (LDI) was used for measuring blood perfusion of the tumor bearing area, which could measure the local blood and vascular network perfusion and reflect the angiogenic activity of the tumor bearing mice indirectly. Our study demonstrated that ursolic acid exerts dual inhibitory effects on both tumor cells and vascular endothelium, significantly suppressing breast cancer growth, metastasis, and angiogenesis, thereby highlighting its potential as a promising anti-metastatic therapeutic agent.
2 Methods
2.1 Cell culture and animals
Human umbilical vein endothelial cells (HUVECs) were cultured in RPMI 1640 medium (Invitrogen, Carlsbad, CA, USA) supplemented with 2 mM glucose, 2 mM glutamine, 100 U/mL penicillin, 100 g/mL streptomycin, and 15% fetal bovine serum (FBS) (1640/15% FBS). Mouse breast cancer 4T1-luc and HEK-293 cells were obtained from the American Type Culture Collection (ATCC, Manassas, VA, USA) and maintained in DMEM (Invitrogen, Carlsbad, CA, USA) containing 10% FBS (Hyclone, Logan, UT, USA) and 50 U/mL penicillin-streptomycin. All cells were incubated at 37 ℃ in a humidified atmosphere with 5% CO2.
The use of female BALB/c mice was approved in accordance with international ethical guidelines and the National Institutes of Health (NIH) Guide for the Care and Use of Laboratory Animals. Five-week-old female BALB/c mice were sourced from Shanghai SLAC Laboratory Animal Co., Ltd. (Shanghai, China) and housed at the Experimental Animal Research Center of Zhejiang Chinese Medical University. All procedures followed NIH guidelines (Publication No. 85-23, revised 1996) for laboratory animal care and use.
2.2 Materials and reagents
Ursolic acid (UA) and doxorubicin (Dox) were obtained from Sigma-Aldrich Chemical (St. Louis, MO, USA). Matrigel was provided by BD Biosciences. Dulbecco's Modified Eagle's Medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin, and trypsin-EDTA were purchased from Invitrogen (Carlsbad, CA, USA). The BCA Protein Assay Kit was acquired from Beyotime. All antibodies were sourced from Cell Signaling Technology (CST, Beverly, MA, USA). Unless otherwise specified, additional reagents were obtained from Sigma-Aldrich.
2.3 Tube formation assay
The tube formation assay was used to evaluate the angiogenic potential of HUVECs. Matrigel (1:1 v/v) was thawed overnight at 4 ℃, then plated onto the bottom of pre-chilled 24-well plates and incubated at 37 ℃ for 1 hour to allow gelation. HUVECs were collected by enzymatic detachment, counted and diluted to 2×105 cell/mL in low-serum medium (1% FBS) containing 20 ng/mL Vascular Endothelial Growth Factor (VEGF) and different concentrations of drugs. Then, HUVECs were seeded on Matrigel and incubated at 37 ℃. Tube formation was observed starting from 8 h after cell seeding and branch points of the capillary-like tubes quantified were countedunder light microscopy (50 × fields).
2.4 MTT assay for cell viability
Cell viability was assessed using the MTT (3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide) assay. 4T1 cells were seeded into 96-well plates at a density of 5 × 103 cells per well and allowed to adhere overnight. Cells were then treated with various concentrations of ursolic acid (UA) (0, 20, 40, 60 μM) for 24, 48, or 72 hours. At each time point, 20 µL of 5 mg/mL MTT solution (Sigma-Aldrich, USA) was added to each well and incubated for 4 hours at 37 ℃. Subsequently, the medium was removed, and 150 µL of DMSO was added to dissolve the formazan crystals. Absorbance was measured at 570 nm using a microplate reader (Bio-Rad, USA). Cell viability was expressed as a percentage relative to the untreated control.
2.5 Cell apoptosis assay by flow cytometry
Apoptosis was evaluated using an Annexin V-EGFP/PI double staining kit (KeyGEN Biotech, Nanjing, China) according to the manufacturer's instructions. Briefly, 4T1 cells were treated with UA (30 μM) for 24 hours, then harvested using trypsin without EDTA, washed twice with cold PBS, and resuspended in 500 µL of binding buffer. Cells were stained with 5 µL of Annexin V-EGFP and 5 µL of propidium iodide (PI) in the dark for 15 minutes at room temperature. The stained cells were analyzed immediately using flow cytometry (FACSCalibur, BD Biosciences). The results were interpreted as follows: Annexin V–/PI– (live cells), Annexin V+/PI– (early apoptosis), Annexin V+/PI+ (late apoptosis), and Annexin V–/PI+ (necrotic cells).
2.6 Cell cycle analysis
Cell cycle distribution was determined by propidium iodide (PI) staining. 4T1 cells were treated with UA (10 μM) for 48 hours, then harvested, washed twice with PBS, and fixed in 70% cold ethanol at 4 ℃ overnight. Fixed cells were washed again and incubated with PI/RNase staining solution containing 50 μg/mL PI and 100 μg/mL RNase A (Beyotime, China) for 30 minutes at room temperature in the dark. DNA content was analyzed using a flow cytometer (FACSCalibur, BD Biosciences), and cell cycle distribution (G0/G1, S, and G2/M phases) was evaluated using FlowJo software.
2.7 In vivo mice xenograft study
4T1-luc cells were treated with 0.25% trypsin and 0.02% EDTA for digestion, followed by centrifugation at 800 × g to collect the cells. The cell pellets were resuspended at 1×107 cells/mL with phosphate-buffered saline (PBS), and 5 ×105 4T1-Luc cells in 100 µL of PBS were injected into the mammary fat pad (MFP) of each mouse using 27 gauge needles. The animals were housed under standard conditions (12-hour light/dark cycle, controlled temperature of 22 ± 2 ℃, and humidity of 50 ± 5%) with ad libitum access to food and water. All animal experiments were performed following the guidelines approved by the Institutional Animal Care and Use Committee (IACUC). Mice were randomly divided into three groups (n = 6 per group): a model group (tumor-bearing mice receiving vehicle treatment), a UA-treated group (tumor-bearing mice administered ursolic acid at 10 mg/kg/day orally), and a Dox-treated group (tumor-bearing mice receiving doxorubicin at 1 mg/kg every other day via intraperitoneal injection) as a positive control. Tumor growth and metastases were monitored by bioluminescence imaging. At the endpoint of the experiment, mice were euthanized humanely by CO2 inhalation according to institutional ethical standards.
2.8 In vivo bioluminescence imaging
Cell implantation and tumor growth were monitored using bioluminescence imaging (BLI) with an IVIS 200 imaging system (Caliper Life Sciences, Hopkinton, MA, USA). Mice were anesthetized with isoflurane and injected intraperitoneally with D-luciferin solution (Gold Biotechnology, St. Louis, MO, USA) at a dose of 100 mg/kg body weight in 0.1 mL of sterile PBS before imaging. Bioluminescence signals were quantified by determining the integrated flux of photons (photons per second) within each area of interest using Xenogen's Living Image V2.50.1 software. Mean ± SE values of photon fluxes were calculated for each experimental point according to time and treatment conditions.
2.9 High-resolution laser doppler perfusion imaging
Laser Doppler perfusion imaging was used to quantify microvascular perfusion in the upper dermis in vivo. This technique is based on the Doppler effect on monochromatic radiation caused by moving erythrocytes in the microvascular network. A laser beam was sequentially moved over the tissue surface, and reflections from moving blood cells were detected by a remote photodiode, converting the returning light into electrical impulses. The signal processor calculated a perfusion signal proportional to the tissue perfusion at each measurement point.
For this study, perfusion images were collected at selected sites: the tumor site (Flux 1), adjacent healthy skin surrounding the tumor (Flux 2), and the heart (Flux 3) in 4T1 tumor-bearing mice [19,20]. Imaging was performed under 1-2% isoflurane anesthesia, with mice maintained at normal body temperature using a circulating water heating pad. Ambient light conditions were standardized across all imaging sessions. To prevent artificial elevation of normal hemoglobin saturation, anesthesia was delivered using 21% oxygen instead of 100% oxygen.
A six-color scale (blue, green, yellow, and red) was used to generate color-coded perfusion images displayed on a monitor. The related perfusion values were calculated as follows:
Tumor perfusion rate = (F1/F3) × 100%
Vascular perfusion rate = (F2/F3) × 100%
2.10 Histopathological analysis and immunohistochemistry
Tumor and lung tissues were fixed in formalin, embedded in paraffin, and sectioned into 5 μm slices. The sections were then deparaffinized in xylene and rehydrated through a graded series of alcohol to distilled water. For histopathological analysis, sections were stained with hematoxylin for 5 minutes and eosin for 2 minutes. For immunohistochemistry analysis, the tissue sections were incubated overnight at 4 ℃ with primary antibodies against ICAM-1, integrin β5, or ESM-1 (Santa Cruz Biotechnology) according to the manufacturer's protocol. The sections were then rinsed three times with PBS and incubated with a biotin-conjugated secondary antibody (Invitrogen) for 30 minutes at 37 ℃, followed by three additional PBS washes. Horseradish peroxidase-labeled streptavidin was added and incubated at 37 ℃ for 30 minutes. The color reaction was developed using 3,3′-diaminobenzidine tetrahydrochloride (DAB), after which the slides were gently rinsed under running tap water. Finally, the sections were counterstained with hematoxylin, dehydrated, dried, and mounted for analysis.
2.11 Statistical analysis
All values are presented as the mean ± standard deviation (SD) of four independent measurements. Statistical analysis was conducted using a two-tailed t-test, assuming two-sample unequal variance. A p-value of < 0.05 was considered statistically significant (* p < 0.05, ** p < 0.01).
3 Results
3.1 Ursolic acid auppresses breast cancer cell proliferation and metastasis
Ursolic acid (UA) significantly inhibited the proliferation of 4T1 breast cancer cells in vitro, demonstrated by the dose-dependent decrease in cell survival rates observed through the MTT assay at 24, 48, and 72 hours post-treatment (Figure 1A). Flow cytometry analysis revealed that UA treatment markedly increased apoptotic cells compared to untreated controls. Specifically, treatment with 30 μM UA for 24 hours induced apoptosis in a substantial population of 4T1 cells, as indicated by increased Annexin V-EGFP and PI staining (Figure 1B). Additionally, cell cycle analysis showed that UA treatment (10 μM for 48 hours) caused a significant arrest of 4T1 cells at the G1 phase, as evidenced by the accumulation of cells in the G1 phase compared with controls (Figure 1C). In vivo experiments further confirmed the inhibitory effects of UA on breast cancer growth and metastasis. Xenogen bioluminescence imaging showed that mice treated orally with UA at 20 mg/kg exhibited significantly reduced tumor growth compared to control mice after 3 and 4 weeks of treatment (Figure 1D). Collectively, these findings suggest that UA effectively suppresses breast cancer cell proliferation and induces apoptosis, alongside inhibiting tumor growth and metastasis in vivo.
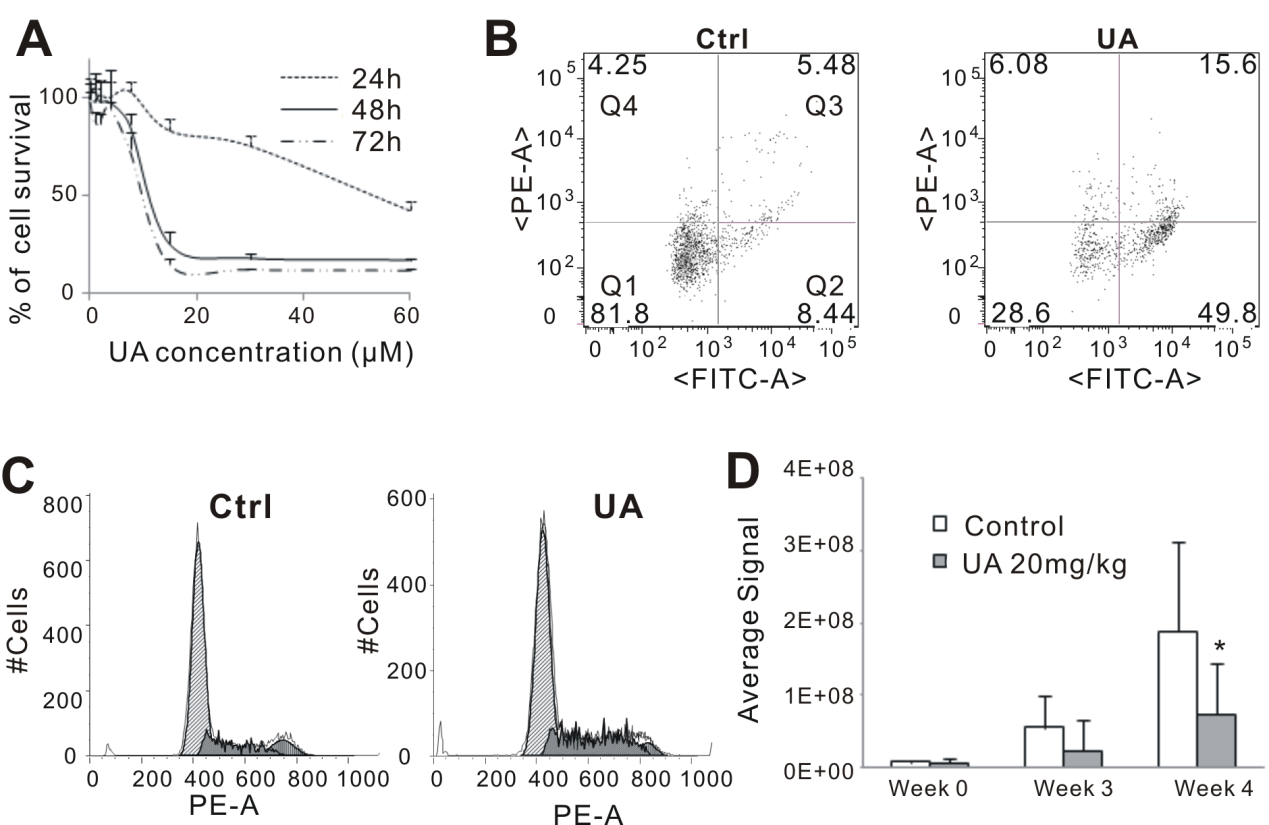
Figure 1 UA inhibits proliferation of breast cancer in vitro and in vivo. (A) MTT assay. 4T1 cells were treated with different concentration of UA for 24, 48 or 72 hours. (B) Cell apoptosis assay. Quadrants: Q1 = live cells; Q2 & Q3 = apoptotic cells; Q4 = necrotic cells. (C) Cell cycle analysis. (D) Quantitative analysis of Xenogen imaging signal intensity (photons/sec/cm2/steradian) after 3 and 4 weeks treatment with UA. * p < 0.05.
3.2 UA inhibits tube formation of HUVECs
We investigated whether UA inhibits angiogenic functions regulated by endothelial cells. The formation of capillary-like structures by endothelial cells on a basement membrane matrix is a well-established method for assessing angiogenesis [18]. To evaluate the role of UA in this process, we conducted an in vitro tube formation assay using human umbilical vein endothelial cells (HUVECs). HUVECs were incubated on Matrigel-coated plates, and tube formation was assessed after 8 hours. As shown in Figure 2, treatment with 10 μM UA resulted in a significant reduction in tube formation, with over a twofold decrease in loop number compared to the control (Figure 2).

Figure 2 UA inhibits tube formation in HUVECs. (A) Quantification of endothelial branch points in a tube formation assay following treatment with VEGF alone (control) or VEGF in combination with UA at 0.1 μM, 1 μM, or 10 μM. Data are presented as mean ± SD (n = 6). ** p < 0.01 vs. control. (B) Representative images of endothelial tube formation under the indicated treatment conditions. Magnification: 100 x.
3.3 UA inhibit tumor blood perfusion
Doppler perfusion imaging (LDPI) is a quantitative technique used for intravital analysis of blood perfusion changes. To assess perfusion dynamics in 4T1 tumor-bearing mice, LDPI was employed to evaluate blood flow in the tumor surface and the surrounding skin. The highest perfusion values were observed in the heart (red), while the tumor site exhibited low perfusion values (blue). Analysis of LDPI images revealed a reduction in abdominal perfusion from week 3 (Figure 3), though no significant difference was observed between the UA-treated and model groups. These findings suggest that UA may slightly improve tumor necrosis four weeks after administration (Figure 3). However, the relative perfusion around the tumor site (Flux 2) in the UA group was significantly reduced to 82.54% of the model group (p < 0.05), indicating that UA markedly decreased local tumor blood perfusion three weeks after administration (Figure 3). This suggests that UA treatment effectively suppressed tumor angiogenesis.
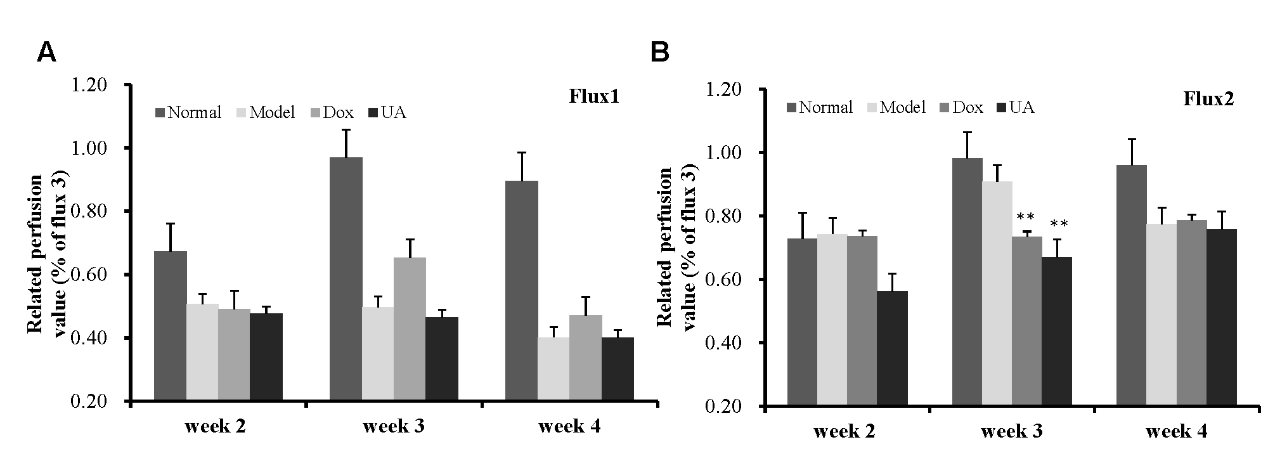
Figure 3 (A–B) UA inhibits in vivo blood perfusion in 4T1 tumor-bearing mice. three examined areas (1.4 * 1.4 cm) covered the tumor (Flux 1), adjacent healthy skin around tumor (Flux 2). Quantification of perfusion values (relative to normal control) measured at weeks 2, 3, and 4 post-treatment in two independent flux regions, Flux1 (A) and Flux2 (B). Groups include Normal, Model, Dox, and UA treatments.
3.4 UA inhibits in vivo tumor metastasis in mouse breast tumor model
To assess the anti-metastatic effect of UA in vivo, 4T1-Luc cells were orthotopically implanted into the mammary fat pads of BALB/c mice. UA was administered orally at 10 mg/kg/day, and doxorubicin (Dox, 1 mg/kg every other day) was used as a positive control. After 28 days of treatment, bioluminescence imaging revealed a marked reduction in tumor burden in the UA-treated group compared to controls (Figure 4A). Histological examination of lung tissues by H&E staining showed a decreased number of metastatic foci in UA- and Dox-treated mice (Figure 4B). Furthermore, ICAM expression in lung sections was reduced in the UA-treated group, as revealed by immunohistochemistry (Figure 4C), suggesting a potential role of UA in suppressing adhesion molecule expression during metastasis.

Figure 4 (A) Representative bioluminescence imaging of mice bearing 4T1-Luc tumors after 4 weeks of treatment with vehicle control (Ctrl), doxorubicin (Dox), or ursolic acid (UA). Regions of interest (ROI) indicate metastatic tumor burden. (B) Hematoxylin and eosin (H&E) staining shows metastatic tumor foci in the lungs (indicated as "T"). (C) Immunohistochemistry for ICAM demonstrates expression levels in lung tissues across different treatment groups. Magnification: 400 ×.
3.5 UA inhibits in vivo angiogenesis in 4T1 tumor bearing mice
Based on the in vitro findings, we next investigated the effects of UA on angiogenesis in vivo using the 4T1-luc tumor-bearing mouse model. Following oral administration of UA and subsequent sacrifice of the mice, to further elucidate the underlying mechanism by which UA inhibits angiogenesis, we analyzed the expression of adhesion - and angiogenesis-related proteins, including integrin β5, ESM-1, and ICAM-1, in the breast cancer tissue of mice (Figure 5). Immunohistochemical staining demonstrated a substantial reduction in the expression of integrin β5, ESM-1, and ICAM-1 in UA-treated tumors compared to the control group, supporting the role of UA in suppressing tumor angiogenesis.

Figure 5 UA inhibits in vivo tumor angiogenesis in mouse breast cancer. Immunohistochemical staining of Integrin β5, ESM-1, and ICAM-1 expression in control (Ctrl), Dox and UA-treated groups. Representative images show protein expression levels in the respective conditions. Magnification: 400 ×.
4 Discussion
In this study, we demonstrated that UA, a naturally occurring pentacyclic triterpenoid, exerts potent anti-metastatic and anti-angiogenic effects in a murine model of breast cancer. Consistent with previous reports highlighting UA's broad pharmacological activities, including anti-inflammatory and anti-tumor effects, our data provide new mechanistic insights into its role in regulating endothelial function and suppressing tumor progression [9,21-23].
Angiogenesis is a critical step in tumor development and metastasis, and targeting endothelial cells remains a promising therapeutic approach [24,25]. Inhibiting angiogenesis has emerged as an effective strategy in treating various solid tumors, and anti-angiogenic agents such as bevacizumab have been incorporated into clinical regimens for breast and colorectal cancers [26]. However, such therapies often face challenges—including acquired resistance due to activation of alternative pro-angiogenic pathways, and off-target toxicities such as hypertension, bleeding, and thromboembolism [27]. Our in vitro data showed that UA significantly inhibited the proliferation of 4T1 cells and tube formation in HUVECs. In vivo, laser Doppler perfusion imaging revealed a marked decrease in tumor-associated blood perfusion following UA administration, further supporting its anti-angiogenic effect.
Our in vitro data demonstrated that UA significantly inhibited 4T1 cells proliferation, and endothelial cells tube formation. These anti-angiogenic effects may potentially involve modulation of the ERK and NF-κB signaling pathways. Since the ERK/MAPK pathway is a well-established downstream effector of VEGF signaling that promotes endothelial proliferation and angiogenesis [28,29]. UA-mediated suppression of this pathway could contribute to its inhibitory effects. Additionally, the NF-κB pathway may also be involved, either as part of a compensatory stress response or through direct regulation, as has been reported in other cell types [30]. These possible mechanisms warrant further validation through pathway-specific inhibition or genetic approaches.
In vivo, laser Doppler perfusion revealed significantly decreased local tumor perfusion after UA administration, supporting the functional relevance of angiogenesis inhibition. Histological analysis further confirmed the anti-metastatic activity of UA in reducing lung metastatic foci in 4T1 tumor-bearing mice. Notably, UA downregulated several key adhesion molecules-ICAM-1, integrin β5, and ESM-1-which are involved in endothelial cell adhesion, vascular permeability, and tumor extravasation. These molecules also play roles in establishing pre-metastatic niches and facilitating interactions between circulating tumor cells and vascular endothelium. Therefore, UA's simultaneous targeting of angiogenesis and adhesion likely contributes synergistically to its anti-metastatic efficacy.
Compared with conventional chemotherapy agents like doxorubicin, which often cause systemic toxicity and non-selective cytotoxicity, UA appears to act through a distinct mechanism that targets the tumor microenvironment rather than tumor cells alone. This feature may provide a therapeutic window for combining UA with standard therapies to enhance efficacy while minimizing toxicity.
Nevertheless, several limitations of this study should be acknowledged. First, the precise upstream regulators of UA's action remain to be fully elucidated, particularly in relation to its dual modulation of ERK and NF-κB signaling. Second, although our model focuses on breast cancer, the applicability of UA to other solid tumors requires validation. Lastly, pharmacokinetic data such as bioavailability, metabolism, and tissue distribution of UA in vivo were not addressed in this study and should be explored in future preclinical trials to assess translational feasibility.
In conclusion, our study identifies UA as a promising candidate for inhibiting breast cancer metastasis by targeting both angiogenesis and endothelial-tumor adhesion mechanisms. These findings underscore the therapeutic potential of naturally derived compounds in modulating the tumor microenvironment and provide a foundation for future investigations into UA-based combination therapies or structural analog development.
5 Conclusion
Our findings demonstrate that ursolic acid effectively suppresses breast cancer metastasis through the inhibition of angiogenesis and endothelial cell function. Mechanistically, UA reduces the expression of key adhesion molecules, limiting tumor vascularization and dissemination. These results suggest that UA may serve as a promising candidate for adjunctive therapy in metastatic breast cancer, particularly in strategies targeting the tumor vasculature and microenvironment. Further studies are warranted to optimize UA delivery, assess its long-term efficacy, and explore its potential in combination with current standard-of-care treatments.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The author declares no conflicts of interest.
Author Contributions
X.J.: Project design, Project administration, Methodology, Writing - original draft; K.H.: Conceptualization, Funding acquisition, Writing - review & editing.
Ethics Approval and Consent to Participate
All animal experiments were approved by the Ethics Committee of Hangzhou City University (No. 15725).
Funding
This study was supported by the Zhejiang Provincial Natural Science Foundation of China (LY19H280010).
Availability of Data and Materials
Data supporting this study are included within the article.
Supplementary Materials
Not applicable.
References
- American Cancer Society. Cancer Facts & Figures 2025. American Cancer Society; 2025.
- Loibl S, Poortmans P, Morrow M, et al. Early breast cancer: ESMO Clinical Practice Guideline for diagnosis, treatment and follow-up. Annals of Oncology 2024; 35(2): 159-182.
- Gradishar WJ, Moran MS, Abraham J, et al. Breast Cancer, Version 3.2024, NCCN Clinical Practice Guidelines in Oncology. Journal of the National Comprehensive Cancer Network 2024; 22(5): 331-357.
- Welt A, Ritter C, Kümmel S, et al. Improved survival in metastatic breast cancer: results from a 20-year study involving 1033 women treated at a single comprehensive cancer center. Journal of Cancer Research and Clinical Oncology 2020; 146(6): 1559-1566.
- Greenberg PA, Hortobagyi GN, Smith TL, et al. Long-term follow-up of patients with complete remission following combination chemotherapy for metastatic breast cancer. Journal of Clinical Oncology 1996; 14(8): 2197-2205.
- Jones SE. Metastatic Breast Cancer: The Treatment Challenge. Clinical Breast Cancer 2008; 8(3): 224-233.
- Weis SM, Cheresh DA. Tumor angiogenesis: molecular pathways and therapeutic targets. Nature Medicine 2011; 17(11): 1359-1370.
- Liu ZL, Chen HH, Zheng LL, et al. Angiogenic signaling pathways and anti-angiogenic therapy for cancer. Signal Transduction and Targeted Therapy 2023; 8(1): 198.
- Ikeda Y, Murakami A, Ohigashi H. Ursolic acid: an anti- and pro-inflammatory triterpenoid. Molecular Nutrition & Food Research 2008; 52(1): 26-42.
- Iqbal J, Abbasi BA, Mahmood T, et al. Ursolic acid a promising candidate in the therapeutics of breast cancer: Current status and future implications. Biomedicine & Pharmacotherapy 2018; 108: 752-756.
- Wang S, Wang N, Tan HY, et al. Ursolic Acid Inhibits Breast Cancer Metastasis by Suppressing Glycolytic Metabolism via Activating SP1/Caveolin-1 Signaling. Frontiers in Oncology 2021; 11: 745584.
- Chan EWC, Wong SK, Chan HT. Ursolic acid: An overview on its cytotoxic activities against breast and colorectal cancer cells. Journal of Integrative Medicine 2019; 17(3): 155-160.
- Yin R, Li T, Tian JX, et al. Ursolic acid, a potential anticancer compound for breast cancer therapy. Critical Reviews in Food Science and Nutrition 2018; 58(4): 568-574.
- Sohn KH, Lee HY, Chung HY, et al. Anti-angiogenic activity of triterpene acids. Cancer Letters 1995; 94(2): 213-218.
- Cárdenas C, Quesada AR, Medina MA. Effects of ursolic acid on different steps of the angiogenic process. Biochemical and Biophysical Research Communications 2004; 320(2): 402-408.
- Gao JL, Shi JM, He K, et al. Tetrandrine Suppresses Cancer Angiogenesis and Metastasis in 4T1 Tumor Bearing Mice. Evidence-Based Complementary and Alternative Medicine 2013; 2013: 265061.
- Bell R, Barraclough R, Vasieva O. Gene Expression Meta-Analysis of Potential Metastatic Breast Cancer Markers. Current Molecular Medicine 2017; 17(3): 200-210.
- Fisher B, Bauer M, Wickerham DL, et al. Relation of number of positive axillary nodes to the prognosis of patients with primary breast cancer. An NSABP update. Cancer 1983; 52(9): 1551-1557.
- Scholz D, Cai WJ, Schaper W. Contribution of arteriogenesis and angiogenesis to postocclusive hindlimb perfusion in mice. Journal of Molecular and Cellular Cardiology 2002; 34(7): 775-787.
- Poole KM, Nelson CE, Joshi RV, et al. Quantitative optical imaging of vascular response in vivo in a model of peripheral arterial disease. American Journal of Physiology-Heart and Circulatory Physiology 2013; 305(8): H1168-H1180.
- Khwaza V, Oyedeji OO, Aderibigbe BA. Ursolic Acid-Based Derivatives as Potential Anti-Cancer Agents: An Update. International Journal of Molecular Sciences 2020; 21(16).
- Kornel A, Nadile M, Retsidou MI, et al. Ursolic Acid against Prostate and Urogenital Cancers: A Review of In Vitro and In Vivo Studies. International Journal of Molecular Sciences 2023; 24(8).
- Sandhu SS, Kumar R, Abbas M, et al. Ursolic acid: a pentacyclic triterpenoid that exhibits anticancer therapeutic potential by modulating multiple oncogenic targets. Biotechnology and Genetic Engineering Reviews 2023; 39(2): 729-759.
- Lugano R, Ramachandran M, Dimberg A. Tumor angiogenesis: causes, consequences, challenges and opportunities. Cellular and Molecular Life Sciences 2020; 77(9): 1745-1770.
- Apte RS, Chen DS, Ferrara N. VEGF in Signaling and Disease: Beyond Discovery and Development. Cell 2019; 176(6): 1248-1264.
- Chen H, Zhang L, Wang Y, et al. Advances in bevacizumab in colorectal cancer: a bibliometric analysis from 2004 to 2023. Frontiers in Oncology 2025; 15.
- Shord SS, Bressler LR, Tierney LA, et al. Understanding and managing the possible adverse effects associated with bevacizumab. American Journal of Health-System Pharmacy 2009; 66(11): 999-1013.
- Okuda KS, Hogan BM, Hall CJ, et al. Live-imaging of endothelial Erk activity reveals dynamic and sequential signalling events during regenerative angiogenesis. eLife 2021; 10: e62196.
- Song YY, Li XH, Li YQ, et al. The role of the ERK signaling pathway in promoting angiogenesis for treating ischemic diseases. Frontiers in Cell and Developmental Biology 2023; 11.
- Shishodia S, Majumdar S, Banerjee S, et al. Ursolic acid inhibits nuclear factor-kappaB activation induced by carcinogenic agents through suppression of IkappaBalpha kinase and p65 phosphorylation: correlation with down-regulation of cyclooxygenase 2, matrix metalloproteinase 9, and cyclin D1. Cancer Research 2003; 63(15): 4375-4383.