Main Text
1 Introduction
Chinese medicine has significantly impacted global public health [1]. Tu Youyou, China's first Nobel Laureate in Physiology or Medicine, isolated artemisinin from wormwood (Artemisia annua), effectively targeting Plasmodium parasites (Plasmodium berghei and Plasmodium cyomolgi) in malaria models. This groundbreaking discovery sparked extensive international interest and research into Chinese medicine [2-4]. Historically, Chinese medicine served as a primary therapeutic system for disease management for millennia before the advent of modern medicine [5]. Numerous empirically validated traditional prescriptions are meticulously documented in classical medical literature, which comprehensively details herbal formulations, therapeutic dosages, preparation methodologies, and clinical applications [6].
Chinese classical prescriptions (CCPs) constitute clinically validated formulations systematically recorded prior to the Qing Dynasty. These prescriptions possess clearly defined therapeutic indications, distinctive pharmacological properties, and established clinical efficacy [7]. The standardization of traditional Chinese medicine has undergone a process from empirical inheritance to modern scientific verification. This provides the technical basis for the patent protection of classic prescriptions [8]. In October 2016, the Central Committee of the Communist Party of China (CPC) and the State Council issued the "Healthy China 2030" strategic outline. This plan outlined specific measures to implement a 15-year roadmap aimed at promoting the preservation, innovation, and widespread adoption of CCPs to enhance public health [5]. In 2018, the Chinese government officially released the initial set of 100 CCPs [9], which included Danggui Buxue Decoction [10], Yiguan Jian [11], Yihuang Decoction [12], and Huangqi Guizhi Wuwu Decoction [13]. These formulations were systematically compiled from 37 canonical medical texts, such as the "Re Bing Lun" and "Thousand Emergency Prescriptions" [14]. The first batch comprised 87 decoctions, 12 powders, and one ointment. Glycyrrhiza uralensis Fisch. (Gancao in Chinese) was the most frequently utilized herb, appearing in 60 CCPs [15], followed by Pinelliae rhizoma (Banxia), found in 17 CCPs [16], and Cinnamomi ramulus (Guizhi), present in nine CCPs [17]. On June 1, 2018, simplified regulatory guidelines for registering CCP pharmaceutical preparations were officially implemented. These guidelines exempted CCP applications from pharmacological studies and clinical trial data requirements, substantially reducing research duration and associated costs [18]. This streamlined regulatory pathway has attracted considerable interest from corporations and research institutions. Subsequently, on September 1, 2023, a second batch of 217 CCPs was officially published, including 93 Han Medicine classical prescriptions, 34 Tibetan Medicine Classical Prescriptions, 34 Mongolian Medicine Classical Prescriptions, 38 Uighur Medicine Classical Prescriptions, and 18 Dai Medicine Classical Prescriptions. Notable examples include Jiegeng Decoction [19] and Mahuang Xixin Fuzi Decoction [20]. Within this batch, Radix bupleuri (Chaihu) appeared in 13 CCPs, while Paeoniae radix alba (Baishao) was included in nine CCPs [21].
While numerous scholars and enterprises have demonstrated interest in the current state of patents related to Chinese classical prescriptions (CCPs), research in this domain remains sparse. This study seeks to delineate the intellectual property landscape of CCPs within the China National Intellectual Property Administration (CNIPA). It provides a detailed examination of patent volume, key inventors, and technological classifications, thereby highlighting the research significance of CCP patents and serving as a valuable reference for researchers, investors, and policymakers.
2 Methods
Using the official list of 317 Chinese Classical Prescriptions (CCPs) issued by the National Administration of Traditional Chinese Medicine, we retrieved corresponding publication numbers and basic patent details through the China National Intellectual Property Administration (CNIPA, www.cnipa.gov.cn). To ensure data quality and completeness, we cross-referenced this information with Derwent Innovation (www.derwentinnovation.com), a global platform providing curated, high-precision patent datasets. Only patents published before March 1, 2025, were included. Data fields collected comprised publication numbers, years, inventor names, patent titles, abstracts, and International Patent Classification (IPC) codes. We first standardized the dataset by eliminating duplicate records arising from abbreviations or misspellings, following the guidelines of the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) [22].
The overall procedure consisted of four key steps: (1) Patents with identical application numbers were manually removed. (2) Patent titles with minor variations were standardized. (3) Inventors were categorized into three groups: (a) Universities/Institutes/Hospitals, (b) Individuals, and (c) Companies. (4) Based on data classification and technological labels, we employed hotspot maps, line charts, cumulative histograms, and matrix analyses to comprehensively illustrate the developmental trajectory and current research landscape of CCP technologies.
3 Results
3.1 Data cleaning and overview
In this study, we defined patent families as inventions filed in multiple jurisdictions but restricted our analysis to Chinese filings. Using data from the CNIPA and Derwent Innovation databases, we identified 1,590 patent entries and removed 172 duplicates to eliminate redundancy. The final dataset consisted of 1,340 unique patents with valid publication numbers (Figure 1 and Table S1).
Among these, the top 10 CCPs accounted for the highest volume of patent filings. Dihuang Decoction led with 74 patents. Seven formulations-Dihuang Decoction, Mahuang Decoction, Xiexin Decoction, Danggui Buxue Decoction, Tanghong Siwu Decoction, Linggui Zhugan Decoction, and Baizhu San—each had over 30 patents (Table 1).
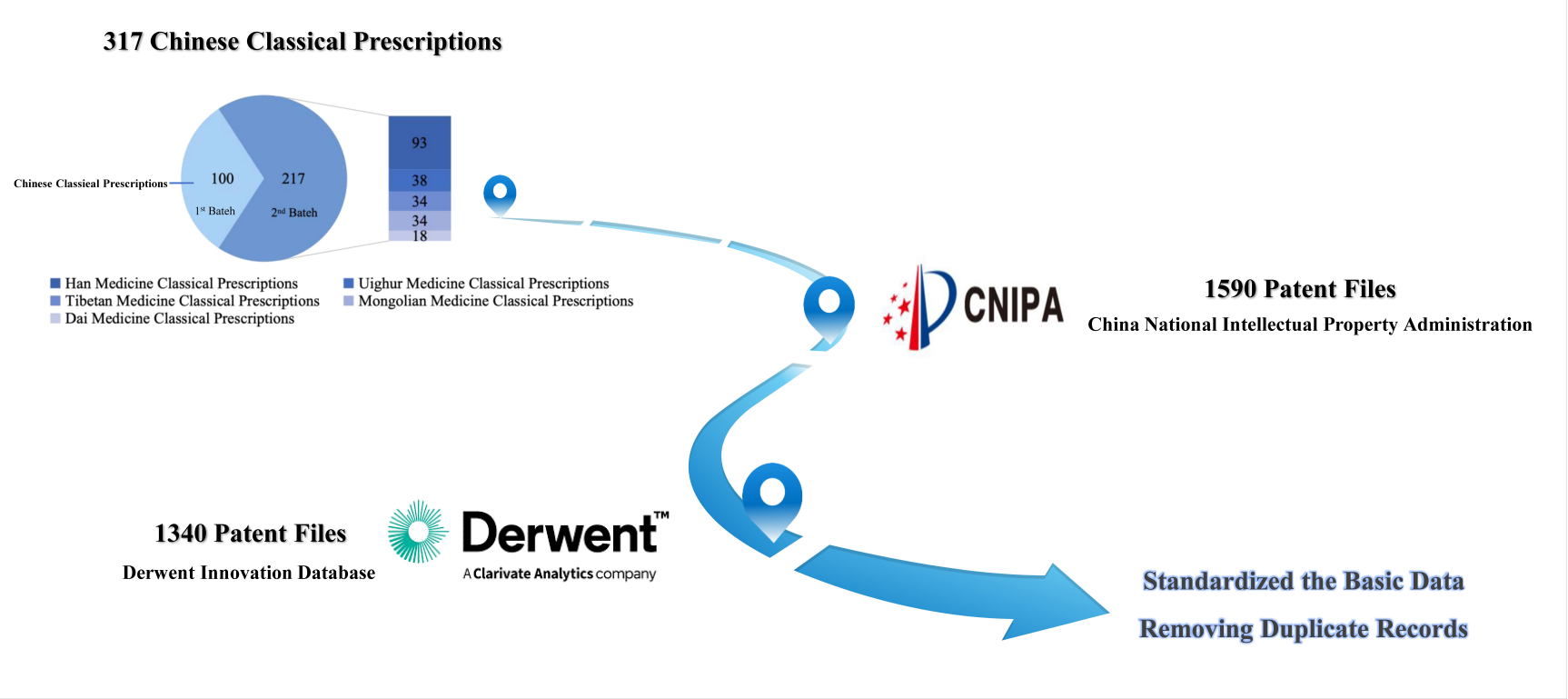
Figure 1 Flow diagram for patent sample selection for Chinese classical prescription (CCP) analysis. Sample selection followed a systematic process of identifying and refining patent data for CCP research.
Table 1 Top classical prescriptions by the number of patents.
| Rank | Classical prescriptions | Number of patents |
|---|---|---|
| 1 | Dihuang Decoction | 74 |
| 2 | Mahuang Decoction | 41 |
| 3 | Xiexin Decoction | 39 |
| 4 | Danggui Buxue Decoction | 37 |
| 5 | Taohong Siwu Decoction | 37 |
| 6 | Linggui Zhugan Decoction | 36 |
| 7 | Baizhu San | 34 |
| 8 | Kaixin San | 28 |
| 9 | Baoyuan Decoction | 27 |
| 10 | Shaoyao Gancao Decoction | 27 |
3.2 Patent application trends
Inventors were primarily concentrated in economically developed coastal regions, including the Yangtze River Delta (24%), the Beijing-Tianjin-Hebei area (18%), and the Pearl River Delta (10%). Notably, South Korea and Japan were the only non-Chinese countries represented among patent applicants (Figure 2).
Patent timeline analysis revealed distinct phases in the development of CCP technologies. Annual CCP patent filings demonstrated fluctuating growth patterns, with sharp increases following major policy interventions, most notably, a 40% rise in 2011 following the release of the national catalog. Since 2019, application numbers have stabilized at approximately 150 filings per year, suggesting a maturation of the regulatory framework (Figure 3A). The median delay between application and publication was 647 days, aligning with standard CNIPA review timelines. Application backlogs peaked between 2015 and 2017, corresponding with a surge in filing volume (Figure 3B).
The interval between patent application and publication serves as a key indicator of the efficiency of the intellectual property system. Shorter publication timelines generally reflect streamlined examination processes and improved operational performance of the patent office. These temporal indicators also provide valuable insights into innovation cycles and the pace of technology transfer efficiency within the CCP sector. From 1985 to 2010, the average publication delay for CCP patents exceeded 1.5 years. Since 2010, however, processing times have gradually decreased, with an average delay of only 35 days recorded by 2025 (Figure 3C).
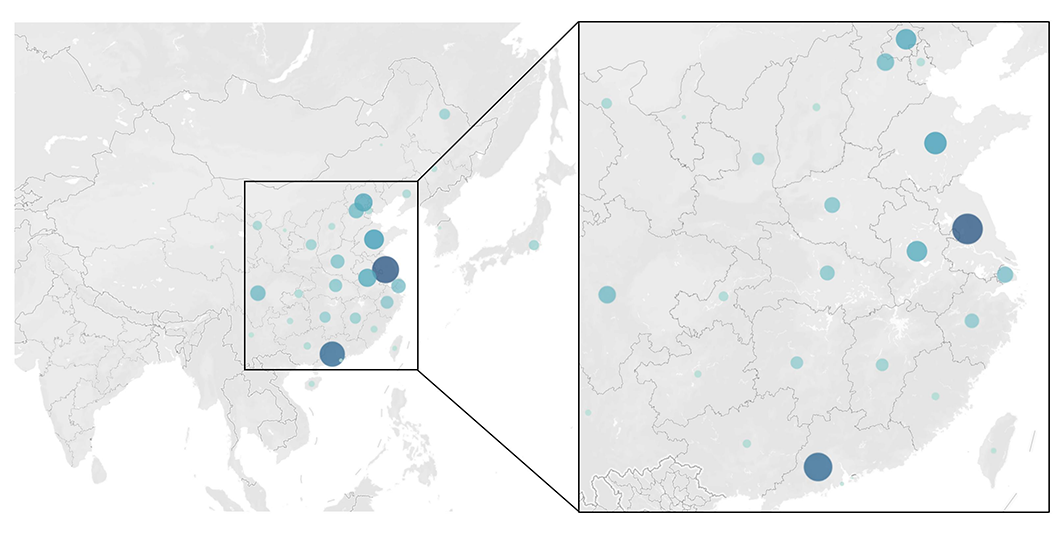
Figure 2 Heatmap visualization of inventor locations, with circle size intensity scaled to the number of application patents.
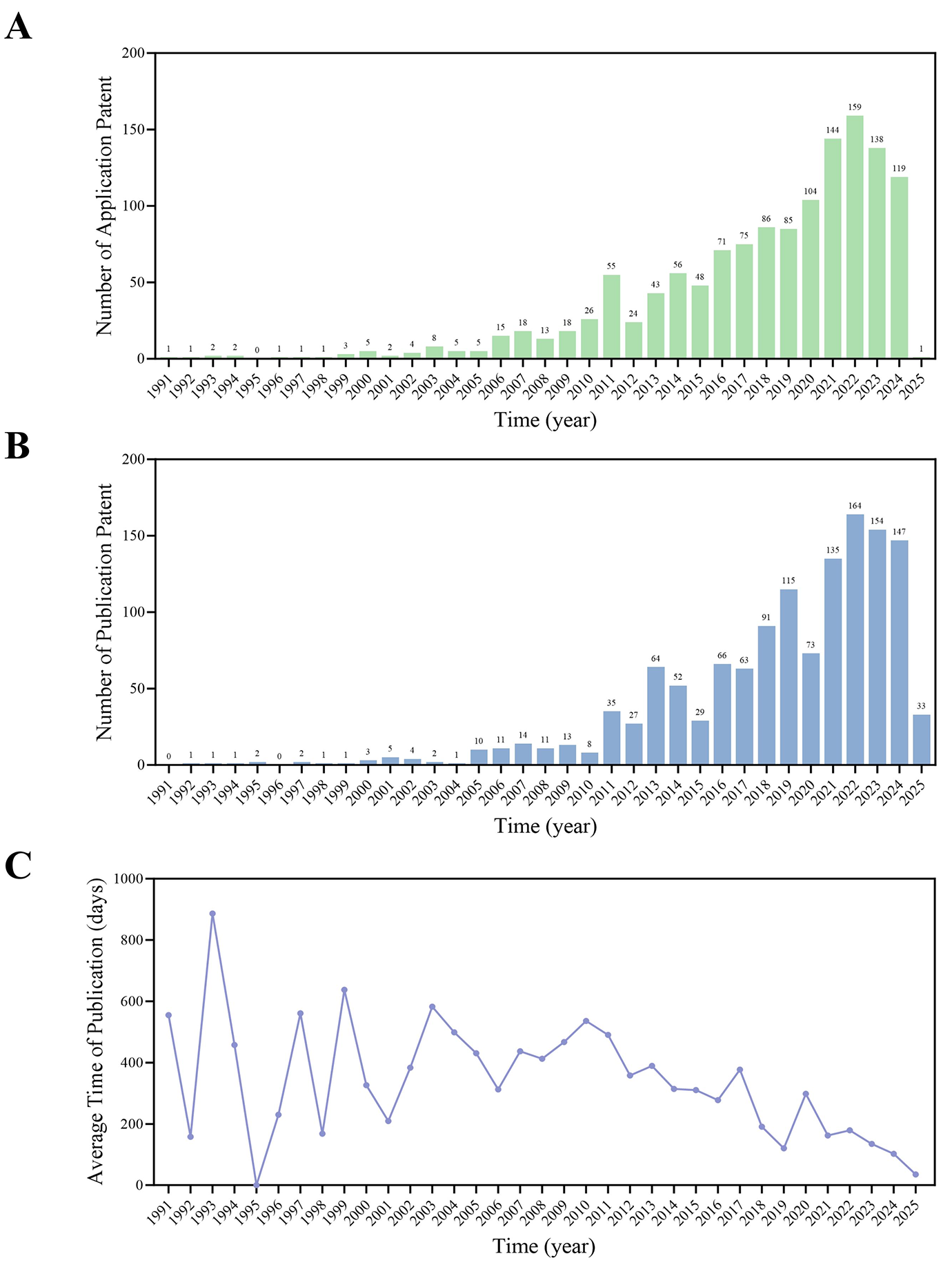
Figure 3 Patent application trend, patent publication trend, and annual approval time of Chinese classical prescriptions (CCPs). (A) CCPs' patent application trend. (B) CCPs' patent publication trend. (C) Annual approval time of CCPs.
3.3 Patent inventors
Among the top 14 CCP patent holders, 10 were companies, three were universities, research institutes, or hospitals, and one was an individual (Table 2). Suzhou Zhiweitang Biotechnology Co., Ltd. emerged as the leading corporate applicant, submitting 50 patents between 2009 and 2011, covering novel dosage forms and preparation techniques for CCPs such as Houpu Mahuang Decoction, Baihe Dihuang Decoction, and Gancao Fuzi Decoction. Nanjing University of Chinese Medicine was the most prolific academic institution, primarily filing patents related to formula enhancement and new therapeutic indications. Academic institutions, serving as the intellectual foundation for CCP innovation, maintained a broader and deeper portfolio of patented technologies compared to industry applicants.
Table 2 Assignees/inventors of top 10 classical prescriptions.
| Rank | Inventor | Number of patents |
|---|---|---|
| 1 | Suzhou Zhiweitang Bio-Tech Co., Ltd. | 63 |
| 2 | Shineway Pharmaceutical Group Co., Ltd. | 41 |
| 3 | Jing-Jin-Ji LianChuang Pharmaceutical Research (Beijing) Co., Ltd. | 35 |
| 4 | Guangdong Yifang Pharmaceutical Co., Ltd. | 22 |
| 5 | China Resources Sanjiu Medical & Pharmaceutical Co., Ltd. | 22 |
| 6 | Sichuan New Green Pharmaceutical Technology Development Co., Ltd. | 19 |
| 7 | Beijing Kangrentang Pharmaceutical Co., Ltd. | 18 |
| 8 | Sinopharm Group Guangdong Global Pharmaceutical Co., Ltd. | 14 |
| 9 | Nanjing University of Chinese Medicine | 14 |
| 10 | Dong-E-E-Jiao Co., Ltd. | 11 |
| 11 | Guangzhou Zhidao Jingfang Technology Co., Ltd. | 11 |
| 12 | Heilongjiang University of Traditional Chinese Medicine | 11 |
| 13 | Shandong University of Traditional Chinese Medicine | 11 |
| 14 | Xiao Mingchun | 11 |
3.4 IPC code characteristics
Each patent's International Patent Classification (IPC) code specifies the technological domain of the invention. Within IPC subgroups, 274 patents (20.52%) were classified under G01N30/02, which pertains to column chromatography techniques used in material analysis (e.g., HPLC systems for the separation of herbal compounds). The second most frequent code, G01N30/06, appeared in 229 patents (17.09%) and covers sample preparation and injection techniques for chromatography (e.g., pre-analytical extraction methods). The third most common code, A61K36/9068, was assigned to 211 patents (15.75%) and refers to herbal medicines derived from Zingiberaceae plants (e.g., ginger-based anti-inflammatory formulations). Fourth, A61P1 appeared in 197 patents (14.70%), encompassing patents focused on analytical methodologies, including quality control and research tools. Fifth, A61P29/00 was associated with 124 patents (9.25%) and relates to therapeutic applications (e.g., clinical efficacy of formulations; Figure 4A). At the subclass level, G01N30 appeared in 1,262 patents, accounting for 94.18% of all filings; A61K36 in 1,177 patents (87.84%); A61K9 in 499 patents (37.24%); and A61P1 in 433 patents (32.31%; Figure 4B).
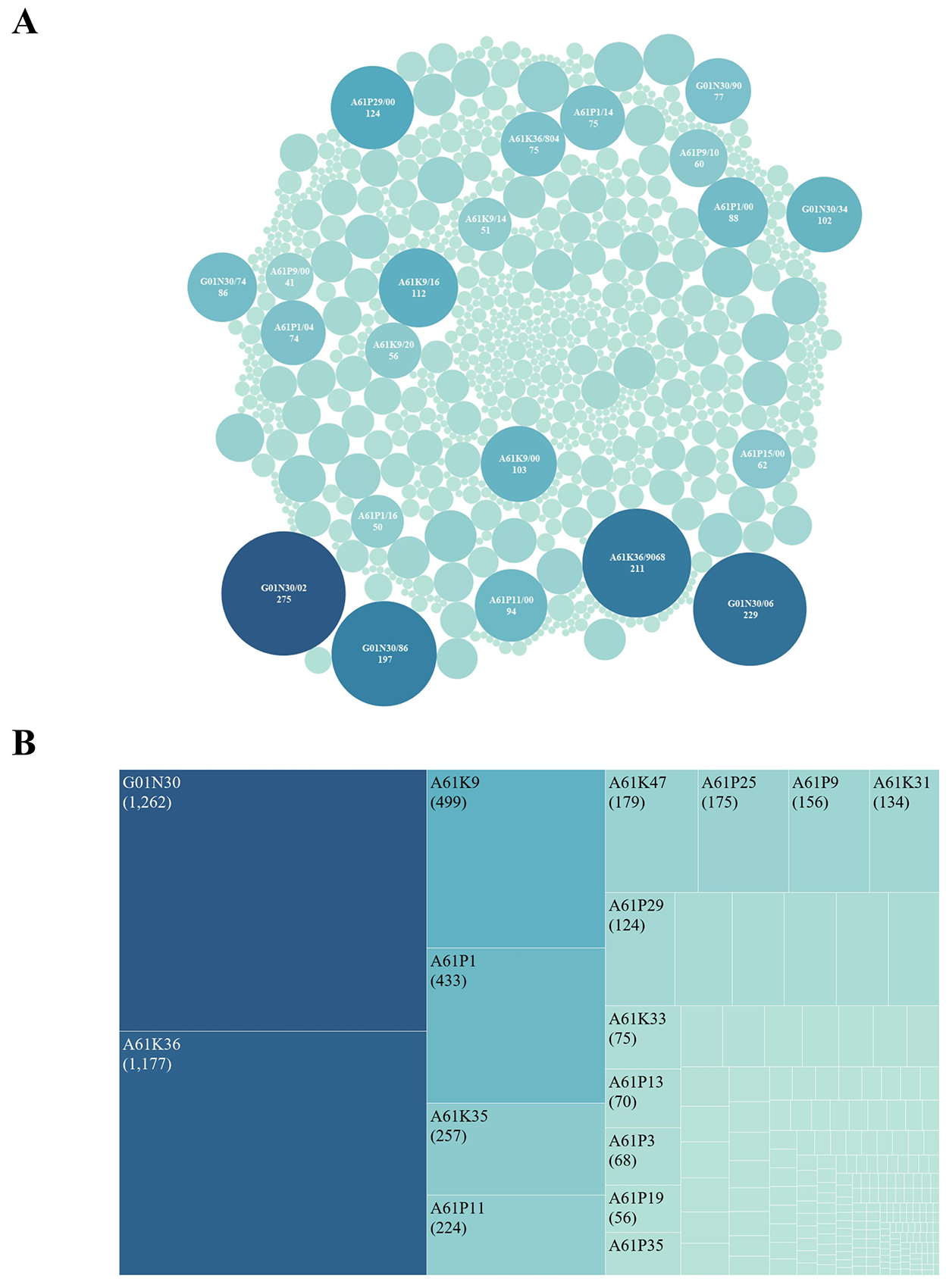
Figure 4 International Patent Classification (IPC) technology for Chinese classical prescription (CCP) patents. (A) IPC subgroup code frequency, with circle size intensity scaled to the number of application patents. (B) IPC subclass code percentage, with square size intensity scaled to the number of application patents.
3.5 Patents technological categories
In terms of subject focus, patents related to preparation methods dominated the portfolio (619 files, 46.2%), followed by improved prescriptions (294 files, 21.9%), analytical methods (267 files, 19.9%), and quality control techniques (240 files, 17.9%). Less frequent categories included new indications (161 files, 12.0%), novel dosage forms (166 files, 12.4%), related equipment (48 files, 3.6%), and miscellaneous topics (62 files, 4.6%). An analysis of 1,340 CCP patents revealed a strong concentration on preparation methods, indicating innovation in extraction, purification, and processing of traditional formulations. The high proportion of improved prescription patents reflects ongoing refinement of herbal compositions for enhanced safety and efficacy (Figure 5A). Notably, a marked increase in filings related to analytical methods and quality control was observed after 2015, signaling a key trend in post-2015 innovation (Figure 5B).
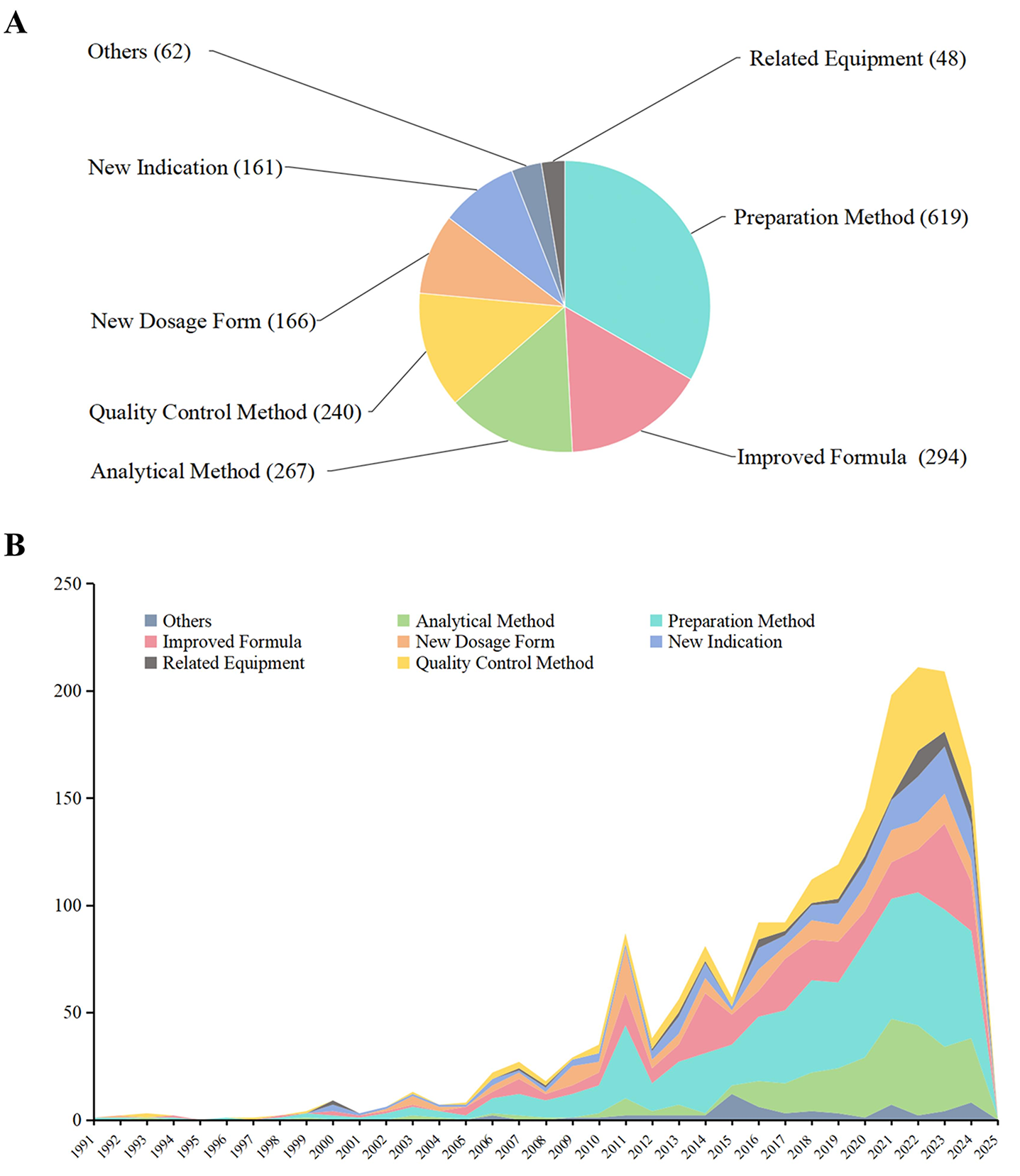
Figure 5 Distribution of patent technology types for Chinese classical prescriptions (CCPs). (A) Pie chart showing the proportion of patents for technological categories. (B) Bar plot showing a trend in the ranking of the same categories.
3.6 Citation network patents
To identify intellectual linkages among key inventions, CCP patents were interconnected based on citation data. The resulting citation network (Figure 6) illustrates clusters of foundational patents and emerging research trajectories within the CCP domain.
Figure 6A illustrates the frequency of internal citations among CCP patents, reflecting how often patents within this domain reference one another. A high density of self-citations signals cumulative innovation, where newer patents build directly on earlier CCP-specific developments. Clustered citation patterns suggest the presence of "patent thickets", in which entities extensively cite their own prior patents to consolidate intellectual property dominance. Notably, the two most frequently cited CCP patents are CN108888748A and CN114441662A, both of which represent pivotal technological advancements in traditional medicine formulation and quality control. CN108888748A addresses critical challenges in standardizing industrial-scale production, while CN114441662A establishes a systematic analytical framework for quality authentication and formula identification. Together, these patents exemplify the transition of traditional empirical formulations into evidence-based, quality-assured therapeutics. Figure 6B extends the citation analysis to include all referenced patents, both CCP and non-CCP. In this broader context, CN119510626A and CN101711814A emerge as the most cited patents, each contributing significantly to the standardization of quality control practices in Chinese medicine.
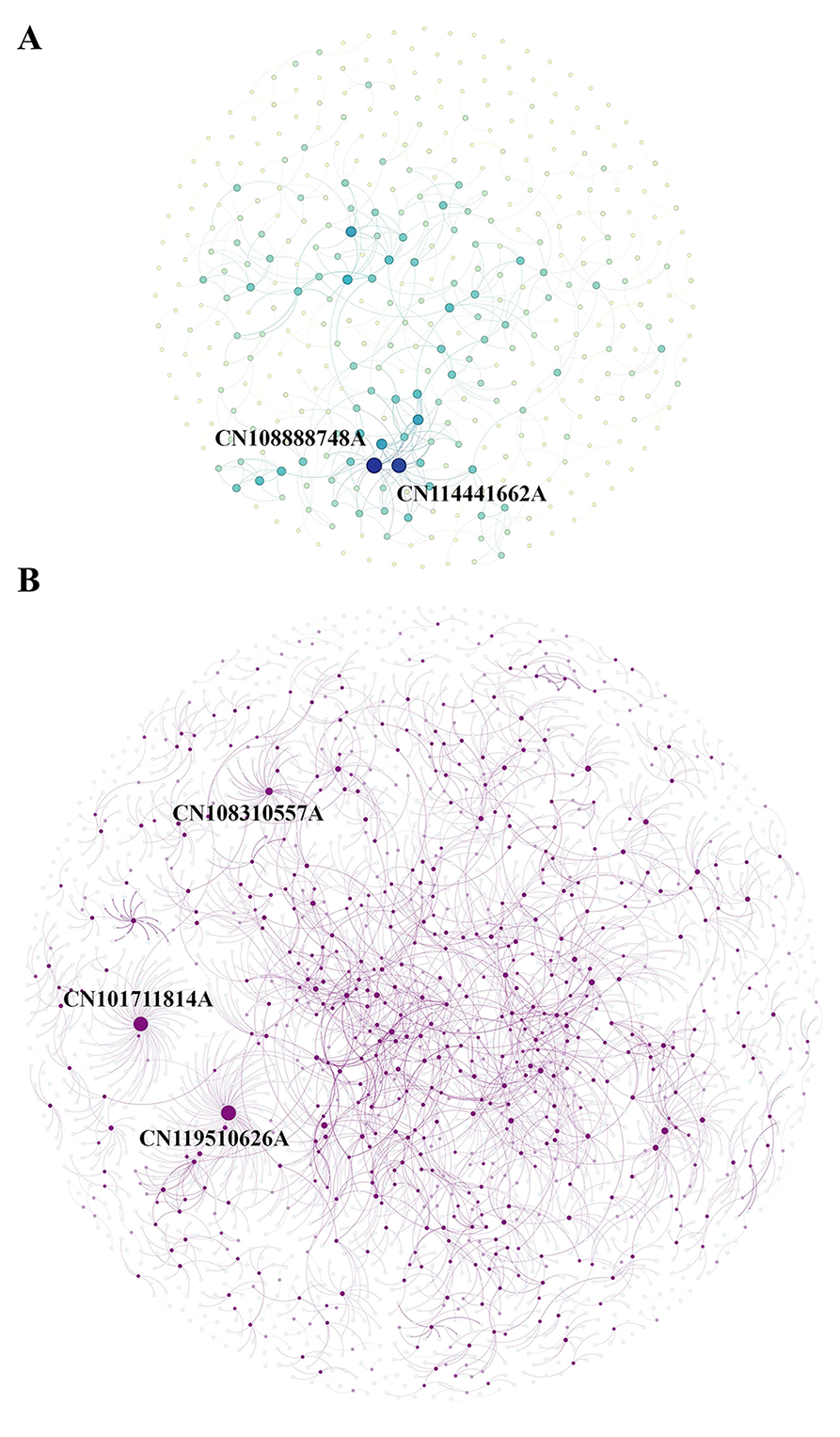
Figure 6 Chinese classical prescription (CCP) citation network. (A) CCPs’ self-cited networks. (B) Whole-citation network. With circle size intensity scaled to the number of application patents.
3.7 Alive/invalid patent
Analysis of patent legal statuses reveals several prominent trends. Substantive examination (259 cases) and rejection (296 cases) were the most common statuses, underscoring the stringent scrutiny CCP patents undergo, likely a result of rigorous examination standards or inadequate application quality. Withdrawal was the most frequent outcome (342 cases), suggesting that applicants often terminate filings strategically, possibly to reduce costs or upon encountering prior art conflicts. Granted patents (325 cases) represent a substantial proportion, indicating a moderate success rate. Meanwhile, maintenance fee lapses (72 cases) point to post-grant sustainability challenges, particularly among smaller applicants. Less frequent legal outcomes, such as avoidance of PCI national phase entry (6 cases) and double patenting rejections (1 case), highlight more specific procedural hurdles (Figure 7A). The granted or invalidated status of patents reflects, albeit imperfectly, their market potential and value. Currently, 325 patents remain active, including a Danggui Buxue Decoction patent that has maintained active status continuously since 2004. However, over 50% of patents filed annually from 2010 to 2019 have since been rendered invalid. Invalidity rates vary by applicant type: 90% of patents filed by individuals, 49% by universities/institutes/hospitals, and 45% by companies have been invalidated. This disparity suggests differing levels of strategic planning, financial sustainability, or technical robustness across applicant categories.
Figure 7B illustrates significant shifts in patent application trends by applicant type in recent years. Corporate entities have markedly increased their patent filings, reflecting a strategic emphasis on research and development (R&D) and intellectual property (IP) protection as mechanisms to sustain competitive advantage. Similarly, universities, research institutes, and hospitals have shown a steady upward trend in filings, highlighting their expanding role in technological innovation, particularly within domains such as biotechnology, healthcare, and advanced materials, which often driven by interdisciplinary collaborations and government-sponsored research initiatives. In contrast, applications from individual inventors have declined, likely due to rising costs, procedural complexities, and heightened examination standards that present challenges for unaffiliated applicants lacking institutional resources.
Figure 7C shows that granted patents continue to comprise the largest share, indicating a consistent rate of successful examination and approval. The volume of pending applications remains substantial, pointing to either procedural delays or the inherently complex nature of the underlying technologies. Invalidated patents represent the smallest proportion, suggesting a relatively high post-grant stability and legal robustness. Collectively, the rise in both granted and pending patents reflects heightened innovation activity, while also implying potential examination bottlenecks within the patent review system (Figure 7C).
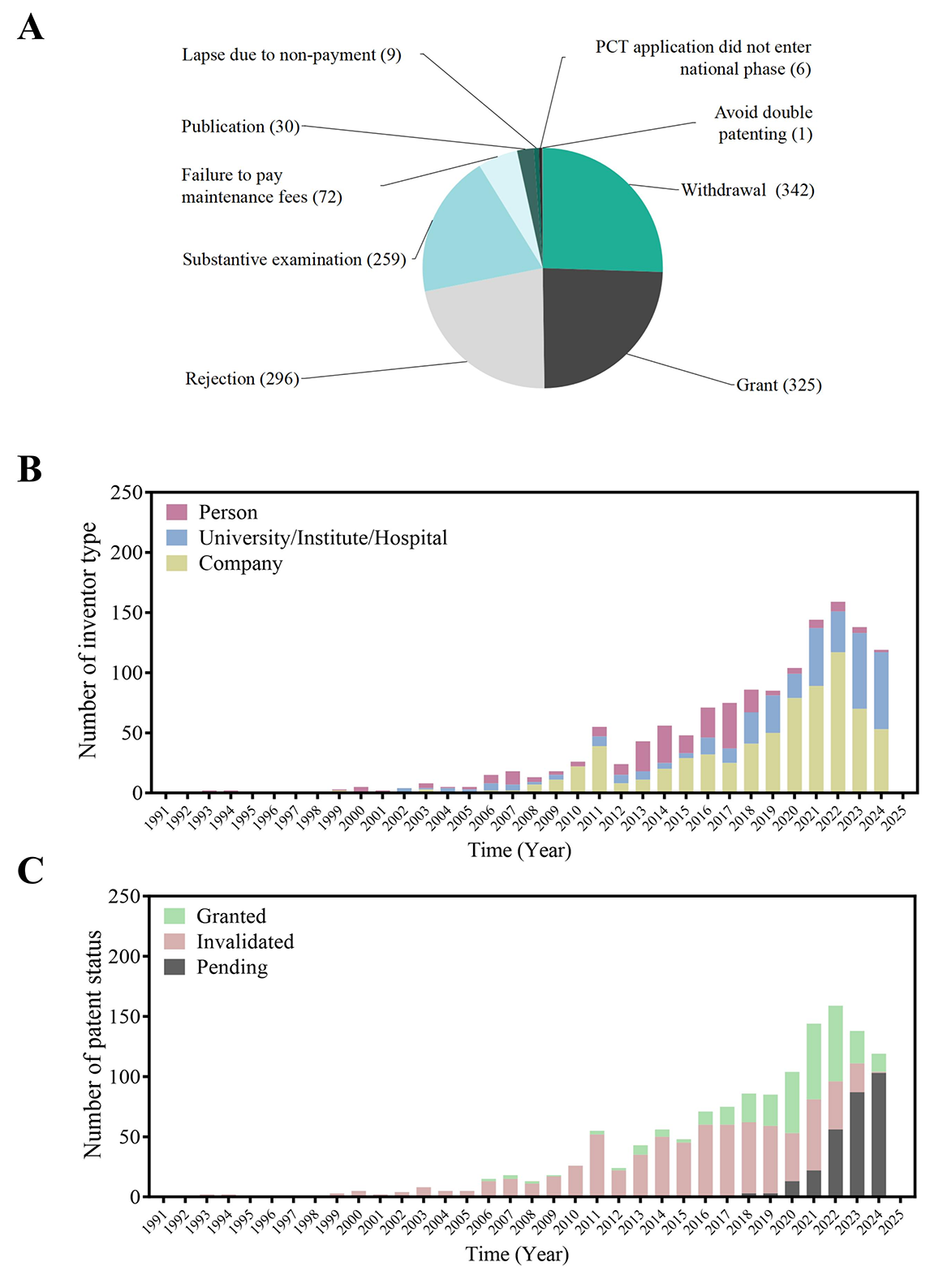
Figure 7 Patent application trends and legal status analysis of Chinese classical prescriptions (CCPs). (A) Distribution of patent legal statuses among CCP-related applications. (B) Patent trends by inventor type. (C) annual legal status distribution.
4 Discussion
4.1 More specific and sustained policies are needed
After submission, the period preceding patent disclosure constitutes the longest waiting phase before patent approval. Historically, prior to 2006, each patent endured an average disclosure interval of at least 800 days. From 2006 to 2008, this interval was reduced to roughly one year. Notably, following China's issuance of the document "Strengthening the Protection of Intellectual Property Rights of Ancient CCPs" in late 2018, disclosure times experienced a significant reduction. Since 2019, the average duration until patent disclosure has shortened dramatically to fewer than 200 days, coinciding with a rapid surge in the overall number of patent applications.
It is noteworthy that although the number of patent applications surged in 2011 and again in 2023, these peaks were immediately followed by sharp declines in subsequent years. While the initial growth reflects the positive influence of policy interventions on the development of Chinese classical prescriptions (CCPs), the challenge lies in ensuring sustained policy support to drive consistent implementation and innovation [23].
Policy directives significantly shape the technological focus of patent activity. Following the release of the first relevant policy, there was a marked increase in applications filed by corporate entities, primarily targeting new dosage forms of various CCPs. However, the issuance of the second policy resulted in a substantial decline in patents for novel formulations, which mandated that dosage forms adhere to classical textual references. Consequently, the focus of innovation shifted toward analytical methodologies. This pattern reveals a reactive innovation environment, in which CCP development closely tracks policy changes rather than being driven by intrinsic research momentum. The emphasis on preparation and analytical technologies aligns with China's broader agenda to modernize traditional Chinese medicine, while the relatively low output of patents for new indications may reflect regulatory constraints associated with reinterpreting classical prescriptions.
Enterprises are the principal agents behind the fluctuations in patent filings, underscoring their acute responsiveness to policy shifts. While such sensitivity can accelerate policy-driven innovation, it also highlights the need for timely, coordinated policy measures to ensure sustained technological advancement in the CCP sector. Overall, although Chinese CCP developers demonstrate strong policy awareness and increasing technological engagement, the long-term vitality of this field depends on the introduction of more targeted, consistent, and forward-looking policy frameworks [24].
4.2 Capital entry and gradual commercialization
Although the Chinese government actively promotes the development of classical Chinese prescriptions (CCPs) through supportive policies, capital recognition remains crucial [25]. Given that profitability is a primary objective for enterprises, this study assessed and forecasted the commercialization trajectory of CCPs by analyzing trends among corporate inventors. During the initial 6-year period of CCP patent applications (2002-2007), individual inventors and academic or hospital institutions predominated. The emergence of corporate inventors began in 2008, marked notably by Beijing Jinfanghua Pharmaceutical Technology Co., Ltd. with patents CN101264276, CN101229306, and CN101284051 for Dayuan Decoction, Guyin Decoction, and Jichuan Decoction, respectively. Subsequently, there has been a consistent annual increase in company-filed patents. Initially, patents were predominantly held by individuals; however, over time, this shifted toward dominance by companies, universities, and hospitals. Notably, fluctuations in patent applicationswere chiefly influenced by corporate inventor activity, particularly the declines observed in 2011 and 2019. In recent years, corporate entities have significantly overtaken universities and research institutes in terms of CCP patent filings, reflecting growing capital interest and signaling a shift toward commercial viability and market-driven development within the CCP sector.
Additionally, the data enables identification of inventors' priority countries or regions, indicating where inventors have chosen to secure patent protections. Typically, inventors apply for patents predominantly in countries or regions with substantial market potential for their products. Notably, patent applications within China have not been exclusively filed by domestic entities; international companies, such as Japan's Tsumura & Co., Ltd., have also actively sought patent protection in China. This underscores the ongoing globalization and attractiveness of the Chinese market in the CCP field [26,27].
With the influx of corporate capital, the CCP field is gaining increasing recognition and is poised to enter a rapid development phase in which companies aggressively pursue new CCP technologies [18]. However, this accelerated innovation may trigger a "patent bubble", wherein a surge of hastily filed patents could lead to widespread invalidations and diminished investment returns.
4.3 Improving patent quality and exploring innovative directions
Within this evolving landscape, several milestone patents have shaped the trajectory of CCP intellectual property. The first CCP patent, CN1232268, marked the formal beginning of the field. Invented by Beijing University of Traditional Chinese Medicine in 2002 and granted on June 13, 2012, it established a precedent for academic engagement in CCP innovation. Notably, Tsumura & Co. Ltd. remains the only overseas entity to file a CCP-related patent: CN103237899, filed in 2011 and disclosed in 2016, which focused on bioassay and pharmacological evaluation methods for the Dajianzhong Decoction in RIN-14B cell cultures. This was followed by CN108779484, filed in 2017 and disclosed in 2018, which predicted administration dosages of the same decoction using the class-phylum to thick-walled phylum ratio in patients' intestinal tracts. These two patents have been extended for protection in seven jurisdictions, including Japan, the United States, and the European Union. Another significant patent is CN100515452, filed by Huang Daju in 2005 and disclosed in 2009, which has the longest granting period. It concerns an oral formulation of the Wuweizi Decoction developed through modern pharmaceutical techniques and is recognized for both its long-term validity and substantial commercial value. Additionally, the most highly cited CCP patent holds foundational importance in the field, although unnamed here. Its frequent citation by subsequent filings underscores its status as a benchmark for innovation and technical originality.
Nevertheless, compared to patents filed by international entities, a disproportionate number of Chinese CCP patents have been invalidated due to limited commercial viability [11,12], a persistent challenge in the field. Despite supportive financial and legislative measures, the CCP patent landscape remains dominated by method patents with relatively low inventive complexity and limited market leverage. As CCPs continue to expand globally, addressing these structural weaknesses will be essential for achieving sustainable and competitive innovation [28].
This study has several limitations. Although patents serve as key indicators of innovation, a patent-centric analysis does not capture the full scope of R&D activities in the CCP field. Nonetheless, based on an extensive review of CCP-related literature, we consider patent analysis a valuable and necessary supplement to existing research. Several important questions remain unanswered and merit future investigation. For example, despite the steady rise in CCP patent applications and the acceleration of R&D, how many of these inventions are both genuinely innovative and commercially viable? Additionally, as a leading example of revitalizing traditional formulations, what is the patent landscape in Japan, and how is it different from that in China? Addressing these questions is crucial for guiding the sustained development and modernization of traditional therapies.
5 Conclusion
Policy plays a pivotal role in shaping CCP R&D, and interest in this area continues to grow annually. With enterprises emerging as the primary applicants, CCPs are increasingly recognized for their commercial potential. Correspondingly, the number of patent filings has risen steadily. These trends suggest that the field is entering a period of rapid and sustained growth. However, this momentum also underscores the urgent need for more targeted and enduring policies, strategies to prevent the formation of patent bubbles, and exploration of novel innovation pathways. Moreover, the current scarcity of high-quality patents could hinder future progress. Proactively addressing these challenges will be essential to securing the long-term vitality of the CCP industry.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
R.S. and Y.H. conceived this new strategy and designed the project; W.H.L., W.Y.L. and L.Z. collected the data; W.H.L., L.Z. and M.J. classified the patent; R.S. and X.H. conducted data analysis and drew graphs; R.S., L.Z. and S.W. wrote the manuscript; Y.W., Y.H. and Z.Z. supervised, funded, reviewed and edited for the project. All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Consent to Participate
No ethical approval was required for this article.
Funding
This study was supported by the Macao Science and Technology Development Fund (FDCT 0123/2022/A, 0008/2023/RIC, 001/2023/ALC, and 005/2023/SKL) and the Research Fund of University of Macau (MYRG-GRG2023-00198-ICMS and SRG2022-00052-ICMS), and the Research Fund of Zhongzhi Pharmaceutical Group (CP-031-2023).
Availability of Data and Materials
Data supporting this study are included within the article.
Supplementary Materials
The following supporting information can be downloaded at: https://ojs.exploverpub.com/index .php/jecacm/article/view/249/sup. Table S1: Patent list of Chinese classical prescriptions (CCPs).
References
- Huang J, Shi R, Chen F, et al. Exploring the anti-hepatocellular carcinoma effects of Xianglian Pill: Integrating network pharmacology and RNA sequencing via in silico and in vitro studies. Phytomedicine 2024; 133: 155905.
- Lyu HN, Ma N, Meng Y, et al. Study towards improving artemisinin-based combination therapies. Natural Product Reports 2021; 38(7): 1243-1250.
- Feng X, Cao S, Qiu F, et al. Traditional application and modern pharmacological research of Artemisia annua L. Pharmacology & Therapeutics 2020; 216: 107650.
- Tu Y. Artemisinin-A Gift from Traditional Chinese Medicine to the World (Nobel Lecture). Angewandte Chemie International Edition 2016; 55(35): 10210-10226.
- Wang WY, Zhou H, Wang YF, et al. Current Policies and Measures on the Development of Traditional Chinese Medicine in China. Pharmacological Research 2021; 163: 105187.
- Zhang Y, Li X, Shi Y, et al. ETCM v2.0: An update with comprehensive resource and rich annotations for traditional Chinese medicine. Acta Pharmaceutica Sinica B 2023; 13(6): 2559-2571.
- Kang Q, He L, Zhang Y, et al. Immune-inflammatory modulation by natural products derived from edible and medicinal herbs used in Chinese classical prescriptions. Phytomedicine 2024; 130: 155684.
- Wang YP, Wang YY, et al. Analysis of the development course of traditional Chinese medicine standardization and recommendations on future work. Guidelines and Standards in Chinese Medicine 2023; 1(1):1-8.
- Shi SY, Zhou Q, He ZQ, et al. Traditional Chinese medicine (Liang-Xue-Di-Huang Decoction) for hemorrhoid hemorrhage: Study Protocol Clinical Trial (SPIRIT Compliant). Medicine 2020; 99(16): e19720.
- Zhang Y, Kang Q, He L, et al. Exploring the immunometabolic potential of Danggui Buxue Decoction for the treatment of IBD-related colorectal cancer. Chinese Medicine 2024; 19(1): 117.
- Lu C, Zhang S, Lei SS, et al. A comprehensive review of the classical prescription Yiguan Jian: Phytochemistry, quality control, clinical applications, pharmacology, and safety profile. Journal of Ethnopharmacology 2024; 319(Pt 2): 117230.
- Zhang M, Liu X, Shi H, et al. Research Progress of Yihuang Decoction and Prediction Analysis on Quality Markers. Journal of Experimental and Clinical Application of Chinese Medicine 2024; 5(1): 1-18.
- Jiang M, Yang L, Zou L, et al. A comprehensive quality evaluation for Huangqi Guizhi Wuwu decoction by integrating UPLC-DAD/MS chemical profile and pharmacodynamics combined with chemometric analysis. Journal of Ethnopharmacology 2024; 319(Pt 3): 117325.
- Zhang H, Yang Y, Zhao C, et al. Evaluation of the chronic oral toxicity of the classical ancient prescription Kai-Xin-San. Journal of Ethnopharmacology 2025; 337(Pt 3): 118931.
- He L, Kang Q, Zhang Y, et al. Glycyrrhizae Radix et Rhizoma: The popular occurrence of herbal medicine applied in classical prescriptions. Phytotherapy Research 2023; 37(7): 3135-3160.
- Liang Z, Wei J, Chan S, et al. Pinelliae Rhizoma: a systematic review on botany, ethnopharmacology, phytochemistry, preclinical and clinical evidence. Chinese Journal of Natural Medicines 2025; 23(1): 1-20.
- Wu S, Wang T, Wu J, et al. Transcriptomic Analysis on the Key Genes and Functional Pathways of Ramulus Cinnamomi in Repressing the Proliferation of Lung Cancer in vitro. Journal of Experimental and Clinical Application of Chinese Medicine 2023; 4(1): 40-52.
- Luo H, Chen H, Liu C, et al. The key issues and development strategy of Chinese Classical Formulas pharmaceutical preparations. Chinese Medicine 2021; 16(1): 70.
- Huang H, Wu D, Li Q, et al. Jiegeng decoction ameliorated acute pharyngitis through suppressing NF-κB and MAPK signaling pathways. Journal of Ethnopharmacology 2024; 332: 118328.
- Yang J, Sun Q, Ma Q, et al. Mahuang Xixin Fuzi decoction ameliorates apoptosis via the mitochondrial-mediated signaling pathway in MCM cells. Journal of Ethnopharmacology 2022; 297: 115538.
- Zhou X, Zeng Z, Fu Y, et al. Exploring the Mechanism of "Radix Bupleuri-Paeoniae Radix Alba" in the Treatment of Post-Stroke Depression Based on Network Pharmacology and Molecular Docking. Journal of Experimental and Clinical Application of Chinese Medicine 2024; 5(4): 82-96.
- Smith JA, Arshad Z, Trippe A, et al. The Reporting Items for Patent Landscapes statement. Nature Biotechnology 2018; 36(11): 1043-1047.
- Zhang Y, Li S, Wu F. Empirical insights into industrial policy's influence on phytoprotection innovation. Frontiers in Plant Science 2023; 14: 1295320.
- Hong G, Shan P. Effects of patent policy on innovation outputs and commercialization: evidence from universities in China. Scientometrics 2018; 117(2): 687-703.
- Annett S. Pharmaceutical drug development: high drug prices and the hidden role of public funding. Biologia Futura 2021; 72(2): 129-138.
- Lyu L, Feng Y, Chen X, et al. The global chimeric antigen receptor T (CAR-T) cell therapy patent landscape. Nature Biotechnology 2020; 38(12): 1387-1394.
- Liu K, Zuo H, Li G, et al. Global research on artemisinin and its derivatives: Perspectives from patents. Pharmacological Research 2020; 159: 105048.
- Wu S, Wang C, Bai D, et al. Perspectives of international multi-center clinical trials on traditional Chinese herbal medicine. Frontiers in Pharmacology 2023; 14: 1195364.