Main Text
1 Introduction
Psoraleae Fructus, the traditional Chinese medicine (TCM), the dried mature fruit of Psoralea corylifolia L., is first recorded in Master Lei's Discourse on Medicinal Processing, and functions in kidney and spleen meridians with pungent and bitter flavors and warm nature. Bavachin (BV), a kind of flavonoids compounds, is the main chemical components extracted from Psoraleae Fructus. BV has the functions of promoting osteogenesis, anti-inflammation, anti-tumor, anti-diabetes, etc., hinting its potential clinical application prospect, and meanwhile, it has the toxicological effects of hepatotoxicity, reproductive toxicity and nephrotoxicity. The various bioactivities of BV have attracted wide attention. This study comprehensively sorted out and summarized the research reports on the pharmacological and toxicological effects of BV to date, providing reference for its in-depth research and rational application.
2 The physicochemical property and identification of BV
BV, also termed 3-Dihydro-7-hydroxy-2-(4-hydroxyphenyl)-6-(3-methyl-2-butenyl)-4H-1-benzopyran-4-one, has molecular formula of C20H20O4, and presents as light yellow powder crystal, with flash point of 202.2 ℃, density of 1.244 g/cm3 and boiling point of 557.8 ℃. BV is soluble in organic solvents such as methanol, ethanol, dimethylsulfoxide (DMSO), but difficult to dissolve in water. The current commonly used separation and detection methods include high-performance thin layer chromatography [1], high-performance liquid chromatography, mass spectrometry, capillary electrophoresis, nitrogen-doped carbon dot fluorescence probe detection [2] and the combination of multiple methods.
3 The pharmacological effects of BV
Reportedly, BV has a wide range of pharmacological activities such as estrogen-like, anti-inflammatory, anti-tumor, immunoregulatory, anti-diabetes and its complications effects.
3.1 Estrogen-like effects
3.1.1 Promoting breast cancer cell proliferation and inhibiting melanin synthesis
BV has a weak phytoestrogenic-like effect and functions by binding to and activating the estrogen receptor (ER). BV can promote confluent human breast cancer cells MCF-7/BOS to proliferate [3], and increase mRNA levels of estrogen response genes, such as pS2 and progesterone-receptor (PR) [4]. In addition, the binding of estrogen and ER can impact melanocyte proliferation, and the activity and quantity of tyrosinase (TYR), a key enzyme in melanin synthesis, thereby mediating melanin synthesis. The safe dose of BV suppresses the role of A375 cells in melanin synthesis [5], denoting that BV exerts estrogen-like effects to repress melanin synthesis.
3.1.2 Anti-osteoporosis and accelerating fracture healing
Currently, estrogen replacement therapy is the dominant scheme for treating postmenopausal osteoporosis. However, the long-term supplementation of estrogen may result in uterine hyperplasia and hypertension, ultimately leading to high-risk events of endometrial and breast cancers [6]. Several reports revealed that BV can stimulate proliferation and viability of osteoblasts cultured in vitro [7,8] and facilitate their mineralization and mature [9]. Also, BV promotes rat chondrocyte proliferation to boost osteogenesis [10], and protects patients with osteoarthritis from inflammation mediated injury via dampening the interleukin-1 beta (IL-1β)-induced activation of the inhibitor of kappa B kinase (IKK)-Inhibitory subunit of nuclear factor Kappa B alpha (IκBα)-nuclear factor kappa-B (NF-κB) signaling pathway in chondrocyte lines [11]. These findings indicated that BV plays a role in driving osteogenesis, preventing osteoporosis and mitigating osteoarthritis. Besides, BV induces primary differentiation of human osteoblasts via enhancing Wnt signaling pathway, and thus prevents estrogen deficiency-caused bone loss in ovariectomized rats [6]. BV can activate Cyclic adenosine monophosphate(cAMP)/protein kinase A(PKA)/cAMP-response element binding protein (CREB) signaling pathway via membrane ER II to promote differentiation of rat bone marrow mesenchyml stem cells into osteoblasts [12]. BV also has potential in fracture healing. It has been documented that in some SD rats undergoing craniotomy to induce brain damage and tibial fractures, brain damage and BV intervention both lead to the increase of vascular endothelial growth factor (VEGF) content and directly promote fracture healing, and medium and large doses of BV has more obvious positive effects on fracture healing [13]. Accordingly, BV can exert estrogen-like effects to boost osteogenesis, reduce bone loss, and accelerate fracture healing, displaying the potential to be a safe estrogen alternative.
3.2 Anti-inflammation effects
3.2.1 Asthma inflammation
Inflammation of asthma is a response to mediation by cytokines from type 2 helper T (Th2) cells, and blocking the production of Th2 cells-related cytokines contributes to asthma treatment. Liang et al. [14] found that BV can downregulate Gata-3 expression and Signal transducer and activator of transcription 6 (STAT6) phosphorylation level, dwindle interleukin (IL)-4 expression in splenic T cells of IL-4-GFP transgenic mice, reduce eosinophil infiltration in bronchial alveoli of asthma models, and effectively inhibit serum IL-4, IL-5, IL-13, and IgE secreted activities, ultimately generating a therapeutic effect on inflammatory diseases such as asthma.
3.2.2 Neuroinflammation
Neuroinflammation is one of the important pathological processes of nervous system-related diseases. Complete or partial deficiency of deubiquitinase A20 can cause spontaneous neuroinflammation [15]. BV can play an anti-inflammation role through suppressing A20 compound-mediated NF-κB signaling [16]. At the same time, BV alleviates LPS-induced microglial inflammation by inhibiting the levels of NO, Tumor necrosis factor (TNF)-α and IL-6 [17]. Recent studies have shown that BV can treat diabetes-induced neuroinflammation by targeting protein kinase C Delta (PKCδ) inhibition of the NF-κB pathway, reducing inflammatory response and oxidative stress, thereby improving the survival and function of diabetic neurons [18]. Therefore, BV is a potential drug for the treatment of neuroinflammation.
3.2.3 Acute kidney injury
Acute kidney injury is a serious complication of sepsis, with a rapid onset and high mortality rate. Ban et al. [19] revealed that BV may attenuate oxidative stress and inflammation by downregulating the protein kinase C beta (PKCβ)/mitogen-activated protein kinase (MAPK)/kruppel-like factor (KLF5) axis, thereby improving lipopolysaccharide (LPS)-induced acute kidney injury.
3.3 Anti-tumor effects
Despite great progresses of modern medicine, including surgical techniques, radiotherapy, chemotherapy, and gene targeted therapy, in cancer treatment, TCM monomers have always been a focus of cancer treatment research. Studies have shown that BV can exert cytotoxic effects on cancer cells by activating apoptosis, inducing ferroptosis, and inhibiting cancer cell proliferation [20-23].
IL-6 is produced by various cells, including tumor cells, and is an important cytokine that represses tumor apoptosis. IL-6 can participate in various vital life activities such as cell proliferation and apoptosis via the Janus Kinase (JAK) signaling pathway and the signal transducer and activator of transcription 3 (STAT3) signaling pathway [24]. Lee et al. [25] indicated that BV suppresses STAT3 promoter activity and STAT3 phosphorylation in IL-6 induced human hepatoma cells (Hep3B). Takeda et al. [21] demonstrated that BV inhibits the proliferation of choriocarcinoma cells by increasing the activity of caspase and regulating electron transport chain complexes and oxidative phosphorylation to change metabolic phenotype; also, BV and paclitaxel can synergistically impact placental choriocarcinoma cells to mitigate cancer, which can be used to counteract paclitaxel induced toxicity.
Different from the caspase-mediated apoptotic form of mitochondrial pathway, ferroptosis is another regulated form of cell death mediated by intracellular iron. Luo et al. [22] pointed out BV attenuates osteosarcoma (OS) cell viability, while increasing cellular ferrous levels, accumulation of reactive oxygen species, malondialdehyde overexpression, and consumption of glutathione. STAT3, overexpression of ferroptosis-related protein solute carrier family 7, member 11 (SLC7A11) and pre-treatment with tumor suppressor protein P53 inhibitor rescued BV-induced OS cell ferroptosis, indicating that BV can trigger ferroptosis of OS cells via STAT3/P53/SLC7A11 axis.
In addition, the latest research results unveiled that BV can inhibit the proliferation of human colorectal cancer cells and induce apoptosis by activating the MAPK signaling pathway. This pathway significantly upregulates growth arrest and DNA damage-inducible protein alpha (Gadd45a) expression, ultimately promoting apoptosis of colorectal cancer cells [23].
3.4 Immunoregulatory effects
The nuclear factor of activated T (NFAT) cells are an inducible transcription factor that can control various functional T cells by forming compounds with various transcription factors during immune responses [26]. Jin et al. [27] found that BV activates NFAT mediated transcription in human T cell lines through in vitro experiments, and that BV and antigen (ovalbumin) treatment in mice enhances the T cell response and the production of antigen-specific antibodies based on in vivo experiments, indicating its role as an immunologic adjuvant and immune regulator.
3.5 Anti-diabetes and its complications effects
BV activates peroxisome proliferator-activated receptor γ (PPARγ), regulates carbohydrate metabolism, and translocates glucose transport protein 4 (GLUT4) by activating protein kinase B (Akt) and AMP activated protein kinase (AMPK) pathways in adipocytes, thus strengthening glucose uptake [28], which denoted that BV may have the potential to treat type 2 diabetes by activating insulin signaling pathways.
Reportedly, BV ameliorates diabetes and its complications. BV can improve diabetic nephropathy via inhibiting oxidative stress and enhancing mitochondrial function [29]. BV combined with psoralen isoflavone A may mitigate type 2 diabetes-induced muscle atrophy by suppressing inflammation and improving mitochondrial function [30]. Moreover, Zhang et al. [18] found that BV can repress NF-κB pathway, reduce inflammatory reaction and oxidative stress, improve the survival rate and function of neurons in diabetes, and ultimately improve the depression-like behavior of mice induced by diabetes through targeted inhibition of PKCδ. These results indicated that BV has great prospects for the treatment of diabetes and its complications.
3.6 Other pharmacological effects
Beyond the above pharmacological effects, BV also has been confirmed to have pharmacological effects against Alzheimer’s disease (AD), Bacteria and viruses, angiosteosis, and non-alcoholic fatty liver disease (NAFLD).
3.6.1 Anti-Alzheimer's disease
AD is an age-related neurodegenerative disease mediated by multiple signaling pathways, with abnormal deposition of amyloid β-protein (Aβ) as one of the main pathological features and a source of toxicity. Through experiments, Xu et al. [17] confirmed that BV partially suppresses LPS-induced neuroinflammation, H2O2-induced nerve damage, and spontaneous aggregation of amyloid β peptide 42 (Aβ42). This finding may provide valuable information for future new drug development and the treatment of AD.
3.6.2 Antibacterial and antiviral
S. aureus infection is greatly detrimental to human health and livestock development, where α-human leukocyte antigen (HLA), as a vital toxic factor, plays a critical role. It has been documented that BV can effectively inhibit the hemolytic activity of HLA at the transcriptional and translational levels. Also, BV can not only alleviate S. aureus induced A549 cell damage in vitro, but also reduce the mortality rate of mouse pneumonia infection, lung bacterial load, and lung tissue inflammation in vivo [31]. Hence, BV has the potential to be developed as a candidate HLA inhibitor against S. aureus. Spring viremia of carp virus (SVCV) is one of the nine fish viruses recognized by the International Office for Animal Disease Control. Cheng et al. [32] proved that BV can block SVCV-induced epithelioma papulosum cyprini (EPC) cell apoptosis to some extent to suppress the early event of SVCV replication, but does not affect SVCV infectivity. Thus, BV may act as a promising drug for SVCV infection. Recent studies have shown that BV inhibits virions by effectively inhibiting the early stages of the viral replication cycle after rotavirus infection, specifically inhibiting the synthesis of rotavirus protein (VP6). These results suggest that BV has strong antiviral activity against rotavirus, inhibits viral replication, and is a candidate for natural treatment against rotavirus infection [33].
3.6.3 Anti-angiosteosis
Angiosteosis is the main complication of cardiovascular disease and chronic renal failure. Autophagy helps maintain a stable internal and external environment of the body and plays an important role in regulating arteriosclerosis. He et al. [34] found that BV protects human aortic smooth muscle cells (HASMCs) from angiosteosis and apoptosis mediated by β-glycerophosphoric acid (β-GP). BV intervention can dose-dependently reduce calcium level and expressions of osteoprotegerin (OPG), osteopontin (OPN), runt-related transcription factor 2 (Runx2) and Bone morphogenetic protein 2 (BMP2) in HASMCs, and plays an anti-angiosteosis role by activating the recombinant Autophagy Related Protein 7 (Atg7)/mammalian target of rapamycin (mTOR) autophagy pathway and inhibiting β-catenin signaling.
3.6.4 Anti-NAFLD
NAFLD is a worldwide health issue. Due to the prevalence of obesity, the incidence rate of NAFLD-related metabolic disorders is increasing rapidly, and there is no approved drug to prevent or treat NAFLD currently. The latest research revealed that BV dampens lipogenesis and induces heat production of lipid and brown subcutaneous adipose tissue to mitigate hepatic steatosis and obesity [35], indicating that BV is the potential drug for NAFLD.
The pharmacological effects of BV are displayed in Figure 1 and Table 1.
Table 1 Pharmacological effects of BV.
| Pharmacological effect | Action of mechanism | Reference |
|---|---|---|
| Estrogen-like effect | (1) Promote proliferation of confluent human breast cancer cells MCF-7/BOS; (2) Have ER ligand-binding activity, increase the expressions of pS2 and progesterone receptor, and decrease ERα expression; (3) Influence melanocyte proliferation and suppress melanin synthesis; (4) BV has the effects of promoting osteogenesis, anti-osteoporosis, and anti-osteoarthritis; (5) BV induces primary differentiation of human osteoblasts by upregulating the Wnt signaling pathway; (6) Activate cAMP/PKA/CREB signaling pathway to promote osteogenic differentiation of mesenchymal stem cells; (7) Boost fracture healing. | [3-13] |
| Anti-inflammation effect | (1) Downregulate Gata-3 expression, STAT6 phosphorylation level, and IL-4, IL-5, IL-13 and IgE expression to treat asthma; (2) Exert anti-inflammatory effects on the nervous system through MAPK and NF-κB signaling transduction; (3) Downregulate PKCβ/MAPK/KLF5 axis to attenuate oxidative stress and inflammation and thus improve LPS-induced AKI; (4) Targeting PKCδ inhibition of the NF-κB pathway. | [14-18] |
| Anti-tumor effect | (1) Inhibit the activity of STAT3 and NF-κB, enhance the activity of caspase and OXPHOS, and mitigate hepatic carcinoma, myeloma, placental choriocarcinoma, etc.; (2) Attenuate osteosarcoma cell viability via STAT3/P53/SLC7A11 axis; (3) Mitigate human colorectal carcinoma via activating MAPK. | [19-25] |
| Immunoregulatory effect | Activate NFAT-mediated transcription and enhance T cell response. | [27] |
| Anti-diabetes and its complications effects | Activate PPARγ, regulate carbohydrate metabolism, and relieve diabetic nephropathy, muscle atrophy and depression. | [18,28-30] |
| Other pharmacological effects | (1) Inhibit Aβ 42 accumulation and mitigate Alzheimer's disease; (2) Effectively weaken the hemolytic activity of HLA, the early event of SVCV replication and strong antiviral activity against rotaviru; (3) Protect human aortic smooth muscle cells from the effects of β-glycerophosphate mediated angiosteosis and apoptosis, and inhibit the expressions of calcification related proteins OPG, OPN, Runx2 and BMP2; (4) Dampen lipogenesis and induce heat production of lipid and brown subcutaneous adipose tissue to mitigate hepatic steatosis and obesity. | [31-35] |
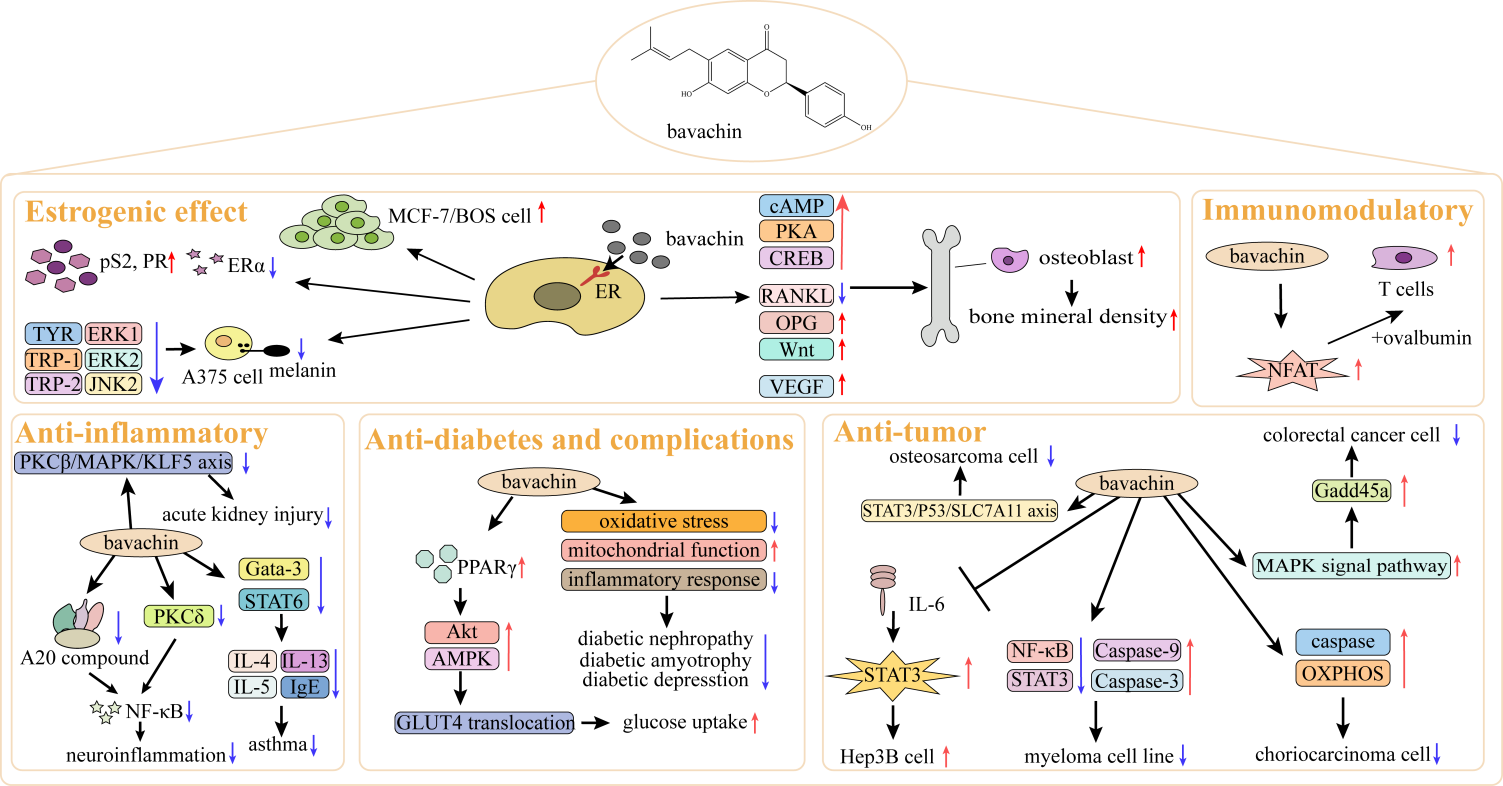
Figure 1 Pharmacological effects of BV.
4 The toxicological effects of BV
4.1 Hepatotoxicity
BV can inhibit the proliferation of normal human hepatocytes (L02) and promote the release of lactate dehydrogenase (LDH), indicating its hepatotoxicity [36]. BV dose-dependently incurs endoplasmic reticulum stress response to activate reactive oxygen species (ROS)/mitofusin 2 (Mfn2)/Akt pathway, thereby leading to cytotoxic reaction [37]. Besides, BV has heterogeneous hepatotoxicity, which increases serum levels of aspartate aminotransferase and alanine aminotransferase in LPS-mediated susceptible idiosyncratic drug-induced hepatic injury mouse models, together with hepatocyte injury caused by increased IL-1β secretion [38]. It has also been reported that the hepatotoxicity of BV may be related to apoptosis mediated by oxidative stress and mitochondrial damage [39].
In the meantime, BV attenuates hepatic drug enzyme activity. CYP2B6 is one of the important drug metabolizing enzymes in the cytochrome P450 (CYP) family, which is widely distributed in the human liver, lungs, kidneys, small intestine, endometrium, and brain, and participates in the synthesis and metabolism of various endogenous and exogenous substances [40]. It has been reported that BV activates the constitutive androstane receptor (CAR) in HepG2 and human colon cancer cell line (LS174T), thereby inducing the mRNA and protein expressions of the target gene CYP2B6 [41].
The recent research uncovered that BV is associated with hepatic immune microenvironment and lipid metabolism. Lin et al. [42] evaluated idiosyncratic hepatic injury induced by BV and epimedin B under immunological stress conditions, and employed lipidomics and multivariate statistical analysis to generate hepatic lipid metabolism profiles. The results revealed that BV combined with epimedin B not only impacts gene related to immune system, but also influences lipid metabolism, resulting in the imbalance of hepatic immune microenvironment and hepatic injury. Therefore, BV or drugs containing BV monomer combined with other drugs may lead to hepatotoxicity as a result of drug interactions.
Moreover, BV damages the normal function of mouse hepatic metabolism. Shen et al. [43] indicated that stearoyl-CoA desaturase 1 (Scd1) may be the central molecule of BV-induced early hepatotoxicity and may be related to the production of polyunsaturated fatty acids (PUFAs). These research results related to BV-induced hepatotoxicity support the prevention and treatment of BV- or Psoraleae Fructus-induced hepatotoxicity.
4.2 Reproductive toxicity
BV has been verified to have reproductive toxicity. BV can accumulate in zebrafish ovaries, giving rise to significant follicular atresia, increased gonadal index and lutein content, and swelling and hypertrophy of the endoplasmic reticulum in the ovaries, accompanied by upregulation of endoplasmic reticulum stress and unfolded protein response related genes. In vitro experiments have suggested that BV can reduce the survival and maturation rate of oocytes [44].
4.3 Nephrotoxicity
Ni et al. [45] indicated that 0.5 μM BV treatment brings about prominent renal fibrosis in zebrafish. BV treated human renal tubular epithelium and zebrafish kidneys present significant epithelial to neurocadherin conversion and enhanced expressions of epithelial mesenchymal transition (EMT) related proteins. BV mainly induces EMT and renal fibrosis via Bip/eIF2α/chop-mediated endoplasmic reticulum stress. Hence, endoplasmic reticulum stress-associated toxic pathway may be potential intervention targets of BV-induced renal fibrosis. Table 2 exhibits the toxicological effects of BV.
Table 2 Toxicological effects of BV.
| Toxicological effect | Action of mechanism | Reference |
|---|---|---|
| Hepatotoxicity | (1) Increase lactate dehydrogenase release rate; (2) Possibly trigger ER stress response through ROS/Mfn2/Akt pathway or AMPK/mTORC1 pathway; (3) Elevate the serum levels of aspartate aminotransferase and alanine aminotransferase; (4) The hepatotoxicity of BV may be related to apoptosis mediated by oxidative stress and mitochondrial damage; (5) Activate constitutive androgen receptor to induce the mRNA and protein expressions of target gene CYP2B6; (6) BV may be related to hepatic immune microenvironment and lipid metabolism. | [36-43] |
| Reproductive toxicity | Induce significant follicular atresia, increase gonadal index and lutein content, and swelling and hypertrophy of the endoplasmic reticulum in the ovaries, accompanied by upregulation of endoplasmic reticulum stress and unfolded protein response related genes. | [44] |
| Nephrotoxicity | BV treatment causes evident renal fibrosis, and BV promotes EMT and renal fibrosis via Bip/eIF2α/chop-mediated endoplasmic reticulum stress. | [45] |
5 Conclusion and Prospects
As a natural small molecular active compound, BV presents broad pharmacological activity, including estrogen-like, anti-inflammatory, anti-tumor, immunoregulatory, anti-diabetes and its complications effects. It can be applied to prevent and treat osteoporosis, cancer and multiple organ-related diseases in the future.
BV is considered as the potential safe alternative of estrogen due to its estrogen-like effects that can promote osteogenesis, reduce bone loss, and boost fracture healing. However, the existing research is relatively superficial and focuses on basic phenomenon, and more in-depth research is of great significance to reveal the specific mechanism of BV promoting osteogenesis and fracture healing. The in-depth studies on the anti-inflammatory, anti-tumor, anti-diabetes and its complications effects of BV contribute to the understanding on the role of BV in inflammation, tumor and diabetes treatment. Besides, BV has the potential for immune regulation, prevention and treatment of Alzheimer's disease, and inhibition of angiosteosis, but relevant research is relatively limited and further exploration is needed.
Psoraleae Fructus is a TCM medicinal material targeting menopausal syndrome, osteoporosis, and vitiligo. In recent years, the occurrence of hepatotoxicity has raised doubts about the safety of TCM medicinal materials. BV, as the primary active components of Psoraleae Fructus, may be related to the hepatotoxicity of Psoraleae Fructus. The current research regarding toxicological effects of BV mainly focuses on the hepatotoxicity, and fewer studies reported its reproductive toxicity and nephrotoxicity. However, there are also studies indicating that BV has potential therapeutic effects on NAFLD and acute kidney injury. Therefore, further research is needed to clarify the mechanism of its toxic effects, and understand the causes for the toxic effects of Psoraleae Fructus and BV, so as to highlight their strengths and avoid their weaknesses, and provide ideas for future drug development and application.
The broad pharmacological effects of BV hint that there are numerous targets of BV. The chemical biology techniques, biophysics, molecular biology techniques, and other methods can be exploited to screen the targets of BV, which combined with gene knockout or gene silencing techniques can be employed to compare the selectivity and activity intensity of BV towards different targets, providing a more comprehensive basis for further research and application of the pharmacological effects of BV.
In conclusion, multi-level and multi-channel in-depth research is required to clarify the pharmacological and toxicological effects of BV, in order to accumulate more theoretical and mechanism support for the in vivo, in vitro and clinical application of BV, as well as promote the efficacy development of BV. The study reviews the research regarding the pharmacological and toxicological effects of BV, in an attempt to provide references for the clinical application as well as development and utilization of BV in the future.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization: Y.J.; Designing and drawing the figures: S.H.; Writing-original draft: Y.J. and J.S.; Writing-review and editing: D.S. All authors have read and agreed to the published version of manuscript.
Ethics Approval and Consent to Participate
No ethical approval was required for this review article.
Funding
Fund projects: General scientific research projects of Zhejiang Provincial Department of Education (Y202351381); Research Fund project of Academy of Chinese Medical Science, Zhejiang Chinese Medicine University (2022Y01).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- Basera IA, Shah MB. A validated high-performance thin-layer chromatography method for quantification of bavachin, bakuchiol, and psoralen from Psoralea corylifolia seeds. JPC - Journal of Planar Chromatography - Modern TLC 2020; 33(3): 293-300.
- Dong Y, Yu X, Wu P, et al. Detection of bavachin based on nitrogen-doped carbon dots as a fluorescence probe. Chinese Journal of Analysis Laboratory 2022; 41(6): 698-703.
- Dong X, Fan Y, Yu L, et al. Synthesis of four natural prenylflavonoids and their estrogen-like activities. Archiv der Pharmazie 2007; 340(7): 372-376.
- Park J, Kim DH, Ahn HN, et al. Activation of estrogen receptor by bavachin from Psoralea corylifolia. Biomolecules & Therapeutics 2012; 20(2): 183-188.
- Wang J, Pei Y, Xu H, et al. Effects of bavachin and its regulation of melanin synthesis in A375 cells. Biomedical Reports 2016; 5(1): 87-92.
- Weng Z, Gao Q, Wang F, et al. Positive skeletal effect of two ingredients of Psoralea corylifolia L. on estrogen deficiency-induced osteoporosis and the possible mechanisms of action. Molecular and Cellular Endocrinology 2015; 417: 103-113.
- Wang D, Li F, Jiang Z. Osteoblastic proliferation stimulating activity of Psoralea corylifolia extracts and two of its flavonoids. Planta Medica 2001; 67(8): 748-749.
- Li W, Yan C, Wu Y, et al. Osteoblasts proliferation and differentiation stimulating activities of the main components of Fructus Psoraleae corylifoliae. Phytomedicine 2014; 21(4): 400-405.
- Kong X, Zhai Y, Liu Y, et al. Effect of bavachin on proliferation and maturation of cranioaural osteoblast in neonatal rats. The Journal of Traditional Chinese Orthopedics and Traumatology 2013; 25(9): 10-15.
- Lee GJ, Cho IA, Kang KR, et al. Biological effects of the herbal plant-derived phytoestrogen bavachin in primary rat chondrocytes. Biological & Pharmaceutical Bulletin 2015; 38(8): 1199-1207.
- Cheng C, Chen Y, Chang W, et al. Phytoestrogen bavachin mediates anti-inflammation targeting Ikappa B kinase-I kappaB alpha-NF-kappaB signaling pathway in chondrocytes in vitro. European Journal of Pharmacology 2010; 636(1-3): 181-188.
- Han Y, Peng Y, Yu Y. Effects of Bavachin on Regulating Differentiation of Bone Marrow MSC by Mediating cAMP/PKA/CREB Signaling Pathway. Chinese Archives of Traditional Chinese Medicine 2019; 37(7): 1597-1600.
- Ji W, Fu Y, Lu W. Effect of Brain Injury and Bavachin on 5-HT and VEGF of Rats with Tibial Fracture. Journal of Emergency in Traditional Chinese Medicine 2014; 23(9): 1585-1588.
- Liang Z, Luo Z, Chen J, et al. Bavachin inhibits IL-4 expression by downregulating STAT6 phosphorylation and GATA-3 expression and ameliorates asthma inflammation in an animal model. Immunobiology 2022; 227(2): 152182.
- Guedes RP, Csizmadia E, Moll HP, et al. A20 deficiency causes spontaneous neuroinflammation in mice. Journal of Neuroinflammation 2014; 11: 122.
- Wang Y, Yang Z, Wang Q, et al. Bavachin exerted anti-neuroinflammatory effects by regulation of A20 ubiquitin-editing complex. International Immunopharmacology 2021; 100: 108085.
- Xu Q, Hu Y, Li G, et al. Multi-target anti-alzheimer activities of four prenylated compounds from Psoralea Fructus. Molecules 2018; 23(3): 614.
- Zhang Z, Sun L, Guo Y, et al. Bavachin ameliorates neuroinflammation and depressive-like behaviors in streptozotocin-induced diabetic mice through the inhibition of PKCδ. Free Radical Biology and Medicine 2024; 213: 52-64.
- Ban KY, Nam GY, Kim D, et al. Prevention of LPS-induced acute kidney injury in mice by bavachin and its potential mechanisms. Antioxidants 2022; 11(11): 2096.
- Takeda T, Tsubaki M, Tomonari Y, et al. Bavachin induces the apoptosis of multiple myeloma cell lines by inhibiting the activation of nuclear factor kappa B and signal transducer and activator of transcription 3. Biomedicine & Pharmacotherapy 2018; 100: 486-494.
- Lee JY, Lim W, Song G. Bavachin suppresses human placental choriocarcinoma cells by targeting electron transport chain complexes and mitochondrial dysfunction. Free Radical Biology and Medicine 2020; 156: 26-35.
- Luo Y, Gao X, Zou L, et al. Bavachin induces ferroptosis through the STAT3/P53/SLC7A11 axis in osteosarcoma cells. Oxidative Medicine and Cellular Longevity 2021; 2021: 1783485.
- Wang M, Tian B, Shen J, et al. Bavachin induces apoptosis in colorectal cancer cells through Gadd45a via the MAPK signaling pathway. Chinese Journal of Natural Medicines 2023; 21(1): 36-46.
- Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. European Journal of Cancer 2005; 41(16): 2502-2512.
- Lee SW, Yun BR, Kim MH, et al. Phenolic compounds isolated from Psoralea corylifolia inhibit IL-6-induced STAT3 activation. Planta Medica 2012; 78(9): 903-906.
- Soto-nieves N, Puga I, Abe BT, et al. Transcriptional complexes formed by NFAT dimers regulate the induction of T cell tolerance. Journal of Experimental Medicine 2009; 206(4): 867-876.
- Jin YH, Kim DE, Jang MS, et al. Bavachin produces immunoadjuvant activity by targeting the NFAT signaling pathway. Phytomedicine 2021; 93: 153796.
- Lee H, Li H, Noh M, et al. Bavachin from Psoralea corylifolia improves insulin-dependent glucose uptake through insulin signaling and AMPK activation in 3T3-L1 adipocytes. International Journal of Molecular Sciences 2016; 17(4): 527.
- Park J, Seo E, Jun HS. Bavachin alleviates diabetic nephropathy in db/db mice by inhibition of oxidative stress and improvement of mitochondria function. Biomedicine & Pharmacotherapy 2023; 161: 114479.
- Yeon MH, Seo E, Lee JH, et al. Bavachin and corylifol a improve muscle atrophy by enhancing mitochondria quality control in type 2 diabetic mice. Antioxidants 2023; 12(1): 137.
- Tao Y, Sun D, Ren X, et al. Bavachin suppresses alpha-hemolysin expression and protects mice from pneumonia infection by Staphylococcus aureus. Journal of Industrial Microbiology & Biotechnology 2022; 32(10): 1253-1261.
- Chen C, Shen Y, Hu Y, et al. Highly efficient inhibition of spring viraemia of carp virus replication in vitro mediated by bavachin, a major constituent of psoralea corlifonia Lynn. Virus Research 2018; 255: 24-35.
- Jung J, Bae J, Park JS, et al. In Vitro Anti-Rotaviral Activity of Bavachin Isolated from Psoralea corylifolia L. (Fabaceae). Veterinary Sciences 2024; 11(5): 188.
- He HQ, Law BYK, Zhang N, et al. Bavachin protects human aortic smooth muscle cells against beta-glycerophosphate-mediated vascular calcification and apoptosis via activation of mTOR-dependent autophagy and suppression of beta-Catenin signaling. Frontiers in Pharmacology 2019; 10: 1427.
- Wei X, Lin L, Yuan Q, et al. Bavachin protects against diet-induced hepatic steatosis and obesity in mice. Acta Pharmacologica Sinica 2023; 44(7): 1416-1428.
- Wang X, Li W, Zhang H, et al. Effect of Psoraleae Fructus and its main components on human normal hepatocyte L02. Traditional Chinese Medicinal Research 2020; 33(4): 59-63.
- Yang Y, Tang X, Hao F, et al. Bavachin induces apoptosis through mitochondrial regulated ER stress pathway in HepG2 cells. Biological & Pharmaceutical Bulletin 2018; 41(2): 198-207.
- Qin N, Xu G, Wang Y, et al. Bavachin enhances NLRP3 inflammasome activation induced by ATP or nigericin and causes idiosyncratic hepatotoxicity. Frontiers of Medicine 2021; 15(4): 594-607.
- Guo Z, Li P, Wang C, et al. Five Constituents Contributed to the Psoraleae Fructus-Induced Hepatotoxicity via Mitochondrial Dysfunction and Apoptosis. Frontiers in Pharmacology 2021; 12: 682823.
- Miksys S, Lerman C, Shields PG, et al. Smoking, alcoholism and genetic polymorphisms alter CYP2B6 levels in human brain. Neuropharmacology 2003; 45(1): 122-132.
- Li H, Wang Y, Ma Z, et al. Inductive effects of psoralen , isopsoralen, bavachin and isobavachalcone cytochrome on P450 2B6. Pharmacology and Clinics of Chinese Materia Medica 2017; 33(1): 15-19.
- Lin M, Li Y, Cao B, et al. Bavachin combined with epimedin B induce idiosyncratic liver injury under immunological stress conditions. Chemico-Biological Interactions 2023; 386: 110774.
- Shen P, Bai Z, Zhou L, et al. A Scd1-mediated metabolic alteration participates in liver responses to low-dose bavachin. Journal of Pharmaceutical Analysis 2023; 13(7): 806-816.
- Huang C, Deng H, Zhou L, et al. Undesirable ER stress induced by bavachin contributed to follicular atresia in zebrafish ovary. Biomedicine & Pharmacotherapy 2023; 166: 115322.
- Ni Y, Deng H, Zhou L, et al. Ginsenoside Rb1 ameliorated bavachin-induced renal fibrosis via suppressing Bip/eIF2α/CHOP signaling-mediated EMT. Frontiers in Pharmacology 2022; 13: 872474.