Main Text
1 Introduction
I-Tiao-Gung means a single radix in Chinese. It is the dried root of the plant Glycine tomentella Hayata, which belongs to the soybean family and wildly grown in Australia and South China. It has been cultivated in Kinman, Taiwan [1], and known as a specialty herbal medicine of Kinman [2].
The 95% ethanol extract of I-Tiao-Gung abbreviated as GTE has been considered analgetic and anti-pyretic in the Chinese traditional concept [3]. The analgesic and anti-inflammatory effects were elucidated in mice [4]. The anti-inflammatory mechanism was related to the decrease in the level of MDA in the mice edema paw via increasing the activities of SOD (superoxide dismutase), glutathione peroxidase, and glutathione reductase in the liver. Commercial products of GTE have been well accepted as a pain-reliever ointment for external use, score patch for reducing sciatica pain. The dried root is also immersed in sorghum liquor as a medicated drink, or in Chinese tea as herbal tea for invigorating blood circulation and eliminating stasis.
2 Methods
Our lab has worked on pharmacological activities of Glycine tomentella, a Chinese herb called I-Tiao-Gung, since 2003, and published 10 papers on this particular topic, in which methodologies were reported [5-13]. A Systematic Review of our science-based studies is performed here to support the traditional concept of Glycine tomentella that invigorates blood circulation and eliminating stasis.
3 Results and discussion
3.1 Active ingredients of GTE
Diadzein was identified the major flavonoid in the leaves and roots of Glycine tomentella Hayata, while gentisic acid and ferulic acid were the major phenolic acids in the leaves. All together 12 phenolic acids and 21 flavonoids were identified [14]. Daidzein was the major compound found in GTE [5,6], followed by daidzin, malonyldaidzin and genistein. They contributed 72.4% of the total GTE. So GTE was an isoflavone-rich extract [7,15]. Other compounds included in GTE were vitexin, malonyl-daidzein, malic acid, and sugars [8].
Total polyphenols of 118 mg gallic acid equivalent/100 g dried root, in which total flavonoids contributed about 23 quercetin equivalent/100 g dried root were quantified in GTE [6].
3.2 Anti-inflammatory activities
GTE exhibited inhibitory effects on the pro-inflammatory enzymes, cyclooxygenase (COX)-2 and lipoxygenase (LOX) [5]. The inhibitory effect on LOX showing IC50 of 16.05 μg/mL, of which a specific fraction dissolved in DMSO improved its inhibitory efficacy to an IC50 of 1 μg/mL. The inhibition of COX-2 by GTE showed an IC50 of 42 μg/mL, while IC50 of daidzein against COX-2 was 14.8 μg/mL. The IC50 of the positive control, indomethacin, was 0.6 μg/mL.
GTE was assessed on the effect observed in a macrophage-like cell line from Atlantic salmon (TO cells), which was stimulated with lipopolysaccharide (LPS) to induce COX-2 and LOX-5, and cytokines including tumor necrosis factor-alpha (TNF-α), and interleukin-1 beta (1L-1β) [7]. LPS induced TNF-α, while GTE at <25 μg/mL inhibited 67-90% of TNF-α gene-expression in TO cells. GTE treatment arrested p38 MAP kinase resulted in accumulation of NADPH and PGE2 by preventing oxidation of NADPH and degradation of PGE2. It was proposed that GTE reduced the pro-inflammatory response in salmon TO cells at an early stage of inflammation (Figure 1).
GTE suppressed the LPS-induced production of IL-1β, IL-6, transglutaminase 2 (TG2) and metalloproteinase-9 (MMP-9) in mouse macrophage cell line RAW264.7 [16], using the same cell line, daidzin and daidzein were found to activate the mitogen-activated protein kinase (MAPK) and nuclear factor kappa B (NF-kB). The signaling pathways may mediate the effect [17]. GTE also inhibited IL-6 in human monocyte cell line U937. GTE enhanced the clearance of apoptotic cells indicating its anti-inflammatory potency and was assessed to be beneficial on treatment of rheumatic diseases [18].
GTE through its active component daidzin effectively improved CYP-induced cystitis by the action of restoring Phase 2 activity and inhibiting the expressions of the receptors [17], and effectively improved cyclophosphamide (CYP)-induced cystitis and CYP-induced oxidative stress, inflammation, and fibrosis through inhibiting the matrix metalloproteinase (MMP)-8, and tissue metalloproteinase (TIMP-1) [17].
More recent in vivo study on guppy (Poecilia reticulata) undergone transport stress, indicated that addition of 1% GTE in packing water significantly inhibited TNF-α and PGE2 genes expression in gill tissues and prevented gill infection (unpublished).

Figure 1 Scheme of proposed mechanism of TNF-α and PGE2 regulation by GTE and LPS in salmonid TO cells [7].
3.3 Anti-oxidative activities
GTE showed in vitro anti-oxidative effects. It inhibited hemoglobin-induced oxidation of linoleic acid. The IC50 was 0.46 μg/mL, while Trolox in DMSO, the positive control, showed IC50 of 6.9 μg/mL [5]. GTE also exhibited DPPH free radical-scavenging activity with IC50 being 18.4 μg/mL, while catechin in methanol served as positive control, the IC50 was 0.088 μg/mL.
The ex vivo lag-phase (△Tlag) of Cu2+-induced human LDL oxidation was significantly (p < 0.01) prolonged and its oxidation rate reduced during the accelerated phase indicating that the GTE served as an antioxidant in plasma, protected the oxidation of LDL in a dose-dependent manner as shown in Figure 2 [9]. A similar inhibitory effect was found on tilapia plasma LDL that GTE inhibited tilapia thrombocyte (nucleated platelet) 5-, 12-, and 15-lipoxygenase (LOX). The IC50 values were 0.43, 0.72, and 0.42 μg/mL, respectively, more inhibitory than that of the positive control, nordihydroguaiaretic acid (NDGA) on the 3 isoforms of LOX (Table 1). The prevention of LDL oxidation and the dual inhibition of LOX and COX-2 is indicative of the possible roles of I-Tiao-Gung in anti-inflammation and anti-atherosclerosis.
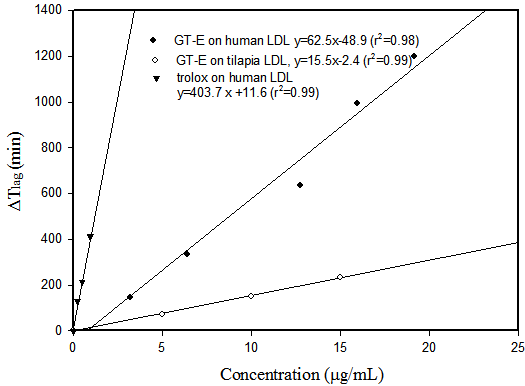
Figure 2 Correlation between GT-E concentration and prolongation of lag phase (△Tlag) of Cu2+-induced oxidation of LDL from human and tilapia using trolox as a positive control.
Table 1 Inhibitory effects on activities of human cyclooxygenase-2 (hCOX-2) and tilapia thrombocyte lipoxygenases (LOXs) by I-Tiao-Gung ethanolic extracts in comparison to indomethacin and NDGA as positive controls.
| Agent | IC50 (μg/mL) | |||
|---|---|---|---|---|
| 5-LOX | 12-LOX | 15-LOX | hCOX-2 | |
| GT-E | 0.43 ± 0.32 | 0.72 ± 0.33 | 0.42 ± 0.28 | 42.0 ± 10.2 |
| NDGA | 2.3 ± 0.3 | 1.6 ± 0.3 | 1.7 ± 0.3 | |
| Indomethacin | 0.61 ± 0.15 | |||
3.4 Hypolipidemia and hypocholesterolemia effects
GTE was shown to have hyperlipidemic effect on hamster [19]. The overfed tilapia showing hyperlipidemia and hypercholesterolemia was intervened with dietary GTE for 8 weeks, exhibited hypolipidemia, and hypocholesterolemia in vivo, especially the plasma LDL-C was reduced in which more tocopherol was retained [10] indicative of GTE in plasma was able to conserve tocopherol inside of LDL by inhibiting the oxidation of LDL which may lead to formation of oxLDL then to form fatty streak in blood vein.
In vitro study showed that GTE had little inhibitory effect on H2O2-induced oxidation [8] indicating the GTE probably would not affect the antioxidant enzymes, i.e., catalase in situ.
3.5 Regulating blood activities
Erythrocytes are the dominant cells in blood. Tilapia fed 1% GTE in feed and injected or exposed to water containing ammonium chloride as stressor produced significantly (p < 0.05) higher levels of highly unsaturated fatty acids (HUFAs) than control, especially more EPA and DHA in the erythrocyte membrane. The ratio of n-3/n-6 was significantly (p < 0.01) higher that the control. Therefore, GTE upregulated the n-3 synthesis pathway leading to inhibited COX-2 activity in erythrocyte membrane. EPA played a crucial role in regulation of PGE2 and inhibition of TNF-α expression [8].
GTE-fed tilapia had significantly (p < 0.01) less blood aggregation and reduced formation of hemoglobin (Hb) dimer (36 kDa) and Hb tetramer (64 kDa) (Lane 3 in Figure 3) than the control (Lane 5 in Figure 3) after exposing to acute temperature drop to 14 ℃ from its optimum temperature being 25 ℃ [8]. In addition, the GTE-fed tilapia showed lower apparent blood viscosity than the control indicative that GTE was protective to erythrocyte membrane structure and blood rheology [8]. This bioactivity of GTE coincides with the traditional concept that GTE enhances blood circulation. This property also partly explains that GTE-fed tilapia adapts to cold stress better, because fish are poikilothermic. The fish body temperature harmonizes with the habitat temperature. When the water temperature drops, lower blood viscosity of fish facilitates blood circulation.
Blood thinning effects of GTE were observed in vitro by mixing with human erythrocytes at hematocrit of 44%. The rheological data fitted the power law model, indicative of the flow behavior becoming closer to Newtonian flow (n = 1) indicative of blood thinning consistency (unpublished). This observation was in line with the inhibition of erythrocyte aggregation properties of GTE which is beneficial to prevent blood clotting.
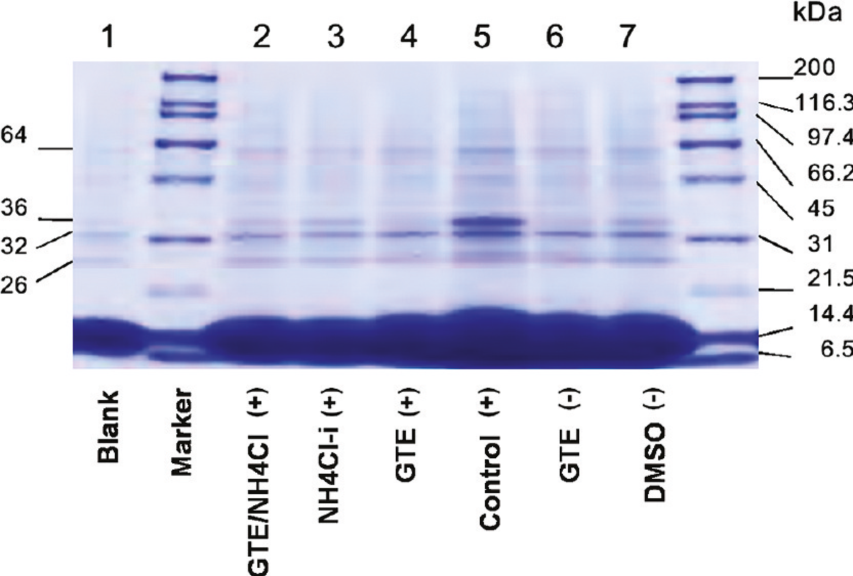
Figure 3 Hemoglobin (Hb) dimer formation is inhibited by GTE. Effects of GTE and NH4Cl on Hb dimer formation in erythrocytes of tilapia exposed to NH4Cl. The 4-20% SDS-PAGE (pH 8.2) gel shows the Hb compounds in hemolysates from tilapia fed with GTE for a period of 3 months prior to injection (NH4Cl-i) and exposure to NH4Cl stress for 2 hours [8].
3.6 Enhancing adaptation to stress
Adding GTE in packing water of orange-spotted grouper (Epinephelus coioides), a high-value food fish, during live transport improved survival rate of grouper. At the same time, packing water deterioration was minimized. The stress indicators, i.e., plasma cortisol, superoxide dismutase (SOD) activity and lipid peroxidation (LPO) were reduced in fish [6]. LPO in plasma showed adversely dose-dependent effect (Figure 4). The packing water maintained lower ammonia-nitrogen and higher dissolved oxygen allowing higher stocking density of grouper at 100% survival. These evidences demonstrated that GTE was able to stabilize the fish metabolism against transport stress and crowding stress.
GTE also down-regulated plasma cortisol, mitigated transport stress of ornamental fishes i.e. blood parrot cichlid (Amphilophus citrinellus × Cichlasoma synspilum) and koi (Cyprinus carpio) thus able to promote fish welfare during live fish transport [11].
Tilapia fed diet containing 1% GTE for 6 weeks followed by sudden cold shock from 25 ℃ to 15 ℃ then dropped to 12 ℃ and maintained for 80 hours then come back to 24 ℃, the survival rate was 100%, while the control was 58.8% (unpublished). GTE enhanced cold tolerance of fish. GTE may be considered a potential adaptogen.
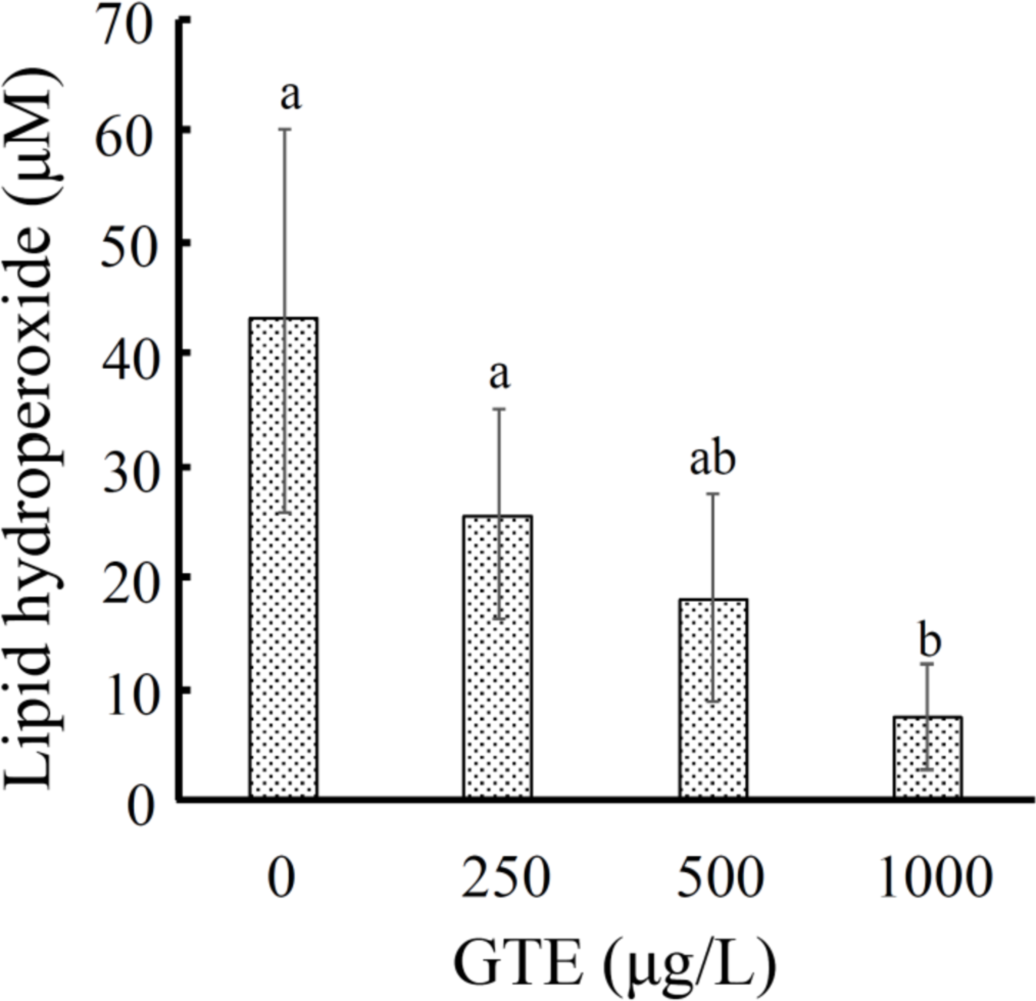
Figure 4 Comparison of the plasma lipid peroxidation level in grouper after 1 hour of simulated transport in water added with GTE [6]. The data were expressed as mean ± standard deviation (n = 3) and analyzed with one-way ANOVA.
4 Conclusion
This review on GTE has showed pharmacological evidences in agreement to the traditional concept that GTE invigorates blood circulation and eliminates stasis using fishes as alternative animal model. GTE also demonstrates its abilities to enhance anti-inflammation, anti-oxidation of plasma LDL and erythrocyte membrane implies that GTE may be used as health food supplement or as an adjuvant to vaccine or antibiotics to improve anti-inflammation in cultured animals or even in human.
The intake of GTE by fishes enhances their ability to adapt to heat or cold stresses or environment pollution, i.e., ammonia in water. The anti-stress property of GTE is much needed by human and cultured animals as global warming and extreme weather are getting more serious than before.
Although the science-based evidences were obtained from fishes, similar effects may also appear in man. Dyslipidemia was found in tilapia fed high-fat diet, and the cardiovascular disease in tilapia was prevented by intervention with freshwater clam extract [12]. Fatty liver diseases were induced by high-fat diet in mice, tilapia and human alike. The hepatoprotective mechanism to alleviate metabolic dysfunction-associated steatohepatitis were found similar except tilapia required 2 weeks to induce the fatty liver symptoms vs. 10 weeks for mice [13]. Therefore, fish is a time-saving and less expensive preliminary animal model to screen bioactivity of herbs. The Glycine tomentella root extract has elucidated the pharmacological efficacy of the historical herbal treatments.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
No conflict of interest with industry or government funding agency.
Author Contributions
B.S.P. and T.-Y.C. wrote the initial paper, B.S.P., T.-Y.C., and W.-L.C. designed and drew the figures, B.S.P. and T.-Y.C. revised the paper.
Ethics Approval and Consent to Participate
No ethical approval was required for this review article.
Funding
This research received no external funding.
Availability of Data and Materials
The analyzed data sets generated during the study are available from the corresponding author on reasonable request.
Supplementary Materials
Not applicable.
References
- Hymowitz T, Hsieh JS. The use of Glycine tomentella Hyata on the islet of Kinmen (Quemoy). Economic Botany 2002; 56(3): 287-289.
- Huang SS, Huang CH, Ko CY, et al. An ethnobotanical study of medicinal plants in Kinmen. Frontiers in Pharmacology 2022; 12: 681190.
- Ko YJ, Hsieh WC, Chiao NY, et al. Pharmacobotanical studies of crude drug I-Tiao-Gung commercially available on the market. Journal of Chinese Medical Sciences 1999; 8(3): 33–46.
- Lu TC, Ko YZ, Huang HW, et al. Analgesic and anti-inflammatory activities of aqueous extract from Glycine tomentella root in mice. Journal of Ethnopharmacology 2007; 113: 142-148.
- Pan BS, Kuo YY, Chen TY, et al. Anti-oxidative and anti-inflammatory activities of two different species of a Chinese herb I-Tiao-Gung. Life Sciences 2005; 77: 2830-2839.
- Wu SM, Tseng YJ, Lin JJ, et al. Mitigation of stress and water deterioration with a root extract of Glycine tomentella during simulated transport of orange-spotted grouper (Epinephelus coioides). Aquaculture 2020; 514: 734485.
- Chuang WL, Haugland Ø, Pan BS, et al. Isoflavone-rich extracts from wooly glycine Glycine tomentella inhibits LPS-induced TNF-α expression in a macrophage cell line of Atlantic salmon (Salmo salar L.). Molecular Immunology 2008; 45: 3956-3964.
- Chuang WL, Pan BS. Anti-stress effect of Glycine tomentella Hayata in tilapia: inhibiting COX-2 expression and enhancing EPA synthesis in erythrocyte membrane and fish growth. Journal of Agricultural and Food Chemistry 2011; 59(17): 9532-9541.
- Chen TY, Shiao MS, Pan BS. Inhibition of 12- and 15-lipoxygenase activities and protection of human and tilapia low-density lipoprotein oxidation by I-Tiao-Gung (Glycine tomentella). Lipids 2005; 40(11): 1171-1177.
- Chen TY, Pan BS. Ex vivo inhibitory effect on tilapia LDL oxidation and hypolipidemia properties of Glycine tomentella root extract. Comparative Biochemistry and Physiology Part A 2007; 148(1): 189-195.
- Wu SM, Chen JR, Chang CY, et al. Potential benefit of I-Tiao-Gung (Glycine tomentella) extract to enhance ornamental fish welfare during live transport. Aquaculture 2021; 534: 736304.
- Huang SC, Lin JJ, Lee MF, et al. Freshwater clam extracts alleviate dyslipidemia of tilapia fed a high-fat diet as an animal model. Journal of Functional Foods 2016; 25: 559-567.
- Lin JJ, Liu YC, Chang CJ, et al. Hepatoprotective mechanism of freshwater clam extract alleviates non-alcoholic fatty liver disease elucidated in vitro and in vivo models. Food & Function 2018; 9(12): 6316-6326.
- Lin JT, Liu SC, Tsay GJ, et al. Composition of flavonoids and phenolic acids in Glycine tomentella Hayata cultivated in various soils. Food Chemistry 2010; 121(3): 659-665.
- Lin SJ, Lay HL, Wu ST, et al. Contents of certain isoflavones in Glycine dolichocarpa, G. tobacina and G. tomntella collected in Taiwan. Journal of Food and Drug Analysis (JFDA) 2005; 13(3): Article 4.
- Tan Y, Zhang X, Cheang WS. Isoflavones daidzin and daidzein inhibit lipopolysaccharide-induced inflammation in RAW264.7 macrophages. Chinese Medicine 2022; 17(1):95.
- Wu KC, Chiang BJ, Tsai WH, et al. I-Tiao-Gung extract through its active component daizin improves cyclophosphamide-induced bladder dysfunction in rat model. Neurourology and Urodynamics 2018; 37(8): 2560-2570.
- Yen JH, Yang DJ, Chen MC, et al. Glycine tomentella Hayata inhibits MMP-9 activity, and enhances RAW264.7 macrophage clearance of apoptotic cells. Journal of Biomedical Science 2010; 17: 83.
- Ko YJ, Wu YW, Lin WC. Hypolipidemic effect of Glycine tomentella root extract in hamsters. The American Journal of Chinese Medicine 2004; 32: 57-63.