Main Text
1 Introduction
Breast cancer is one of the most common tumors in women, the incidence of which is relatively high globally, as reflected by nearly 250,000 newly diagnosed female patients each year, and approximately 40,000 females succumb to breast cancer [1]. Increasing researches pointed out that local treatment brings little effect in prolonging the survival time of patients with metastatic breast cancer [2]. In recent years, macroscopically adjusting the hematopoietic and immune environment of the body through immune antibodies represented by programmed death-ligand 1 (PD-L1) or non-myeloablative allogeneic peripheral-blood stem cell transplantation has achieved remarkable results in effectively blocking the development of tumors.
Massive proliferation of Myeloid-derived suppressor cells (MDSCs) and immature myeloid cells (IMCs) in tumor-bearing body is manifested by extramedullary hematopoiesis (EMH) of spleen, and pathological changes, such as splenomegaly, can be observed. Statistics showed that 32.6% of patients with splenomegaly result from tumor [3]. According to our data and previous findings, breast cancer can secrete G-CSF to induce IMCs to migrate to the EMH organ represented by the spleen, proliferate, and ultimately differentiate into MDSCs, which enter into peripheral blood and tumor tissues after activation, and lead to the formation of an immunosuppressive environment in tumors. Therefore, controlling the abnormal EMH, as well as impeding and even blocking the proliferation and differentiation of IMCs into MDSCs can directly impact the formation of an immunosuppressive environment in breast tumors.
MDSCs are one of the main components of tumor microenvironment, especially in breast cancer, with the immunosuppressive property that can effectively promote the immune escape of malignant tumor cells and thus evidently reduce the therapeutic amount and efficacy of immunosuppression [4]. The accumulation of MDSCs is positively correlated with the clinical classification of breast cancer, and the higher the proportion of MDSCs in the blood of tumor patients, the worse the tumor prognosis [5]. Hematopoietic stem cells can produce IMCs from eukaryocytes, and IMCs can further differentiate into MDSCs which are precursors of neutrophils, dendritic cells and macrophages. IMCs are the primary source of MDSCs in bone marrow, which, under pathological conditions, are prevented from differentiation, remain in the primitive stage and become the MDSC population with immunosuppressive function [6]. Under normal condition, IMCs are produced and present within the human bone marrow, and lack certain immunosuppressive activity.
However, various pathological conditions, including malignant tumors, assorted infectious diseases, sepsis or some autoimmune diseases, may cause the differentiation of IMCs to be blocked, and the accumulation of IMCs in spleen, bone marrow and peripheral blood can result in massive proliferation of MDSCs [7]. Notably, MDSCs have been proven to be associated with poor prognosis of breast cancer patients [8,9]. Serafini et al. [10] firstly successfully provide MDSC models that are related to tumorigenesis and cell proliferation. In the early development stage of model construction, the involvement of IMC diffusion, which is related to the suppression of MDSC terminal differentiation by tumor cells, is widely considered to be triggered by constantly MDSC-produced growth factors (such as granulocyte-macrophage colony-stimulating factor GM-CSF, and granulocyte colony-stimulating factor (G-CSF)) in tumors. The second development stage of model construction refers to diffusion and transformation of IMCs into MDSCs through pro-inflammatory growth factors (IL-1β, IL-6 and TNF-α). Importantly, IMC activation in normal human pathological immune state elevates levels of factors that cause immunosuppressive reactions, including arginase 1 (ARG-1), inducible nitric oxide synthase (iNOS), nitric oxide (NO) and reactive oxygen species (ROS) [11,12]. Newly produced MDSCs act as an immunosuppressor by kinases-produced antibodies of metabolic substances, such as ARG-1, iNOS and ROS. Therefore, suppressing IMC proliferation is an effective method to impede the production of MDSCs.
Rhizoma Zedoariae is the dry root of zingiberaceae Curcuma phaeocaulis Val., Curcuma kwangsiensis S.G. Lee et C.F. Liang in Guangxi province, or Curcuma wenyujin Y.H. Chen et C. Ling, the application history of which was first published in the Theory of Medicinal Properties. Rhizoma Zedoariae has the functions of promoting qi, dredging blood stasis, eliminating accumulation and relieving pain. Previous researches have unveiled two main components of Rhizoma Zedoariae, namely volatile oils and curcumins with anti-tumor property [13,14]. Our previous research shown that, XuanhusuoPowder (composed of two herbs, Zedoary Turmeric and Yuanhu) inhibits myeloid-derived suppressor cells differentiation in the spleen of breast cancer mice by down-regulating G-CSF [15]. There is a dearth of available reports about suppression of IMC proliferation by Chinese medicine, and mechanism of Rhizoma Zedoariae extract and its active components impacting G-CSF-mediated signal transducer and activator of transcription 3 (STAT3) signaling pathway. Hence, this study conducted a novel in vitro assay to mimick the proliferation and differentiation progress of normal myeloid cells into MDSCs, to investigate whether Rhizoma Zedoariae extract and its active components could inhibit the cell viability of THP-1, as well as their ability to inhibit the THP-1 differentiate into MDSCs, which induced by breast caner cells through G-CSF/STAT3 signaling pathway.
2 Materials and methods
2.1 Cell lines
Human leukemia monocytic cell line THP-1 was ordered from the Shanghai Cell Bank of Chinese Academy of Sciences (No. SCSP-567, Shanghai, China).
2.2 Drugs and reagents
Rhizoma Zedoariae was purchased from outpatient department of Zhejiang Chinese Medical University in Binjiang district, which has been authenticated by HE Kai, the chief pharmacist of Chinese medicine from Pharmacy of Traditional Chinese Medicine, the First Affiliated Hospital of Zhejiang University. Rhizoma Zedoariae and 75% alcohol (1:5 ratio) were heated, refluxed and extracted thrice. Then, the extract was subjected to vacuum concentration under reduced pressure, prepared into liquid extract (the yield was 11.22%), and stored at -20 ℃. DMEM high-glucose culture solution (8118397, Gibco, Waltham, MA, USA), dimethyl sulfoxide (DMSO, BB02BA001, Sangon Biotech, Shanghai, China), 3-(4,5-dimethylthiazol-2-yl)-2, 5-diphenyltetrazolium bromide (MTT) (EZ2811D347, Biofroxx, Guangzhou, China), 2×SG fast qPCR kit (D927KAC925, Sangon Biotech, Shanghai, China), cDNA reverse transcription kit (DB08KA7000, Sangon Biotech, Shanghai, China), Trizol Reagent (15596018, ThermoFisher, Waltham, MA, USA), Triton X-100 (ST795, Beyotime, Shanghai, China), chloroform (20161215, Xilong Chemical, Beijing, China), isopropanol (20160321, Zhedong Special Chemical Reagent Factory, Linhai, China) and ethanol (20201225, Sinopharm Chemical Reagent Co., Ltd., Shanghai, China) were procured in advance. Curcumin, germacrone, curcumol, curcumenol and curdione (purity > 98%) were purchased from Shanghai Tauto Biotech Co., Ltd. (Shanghai, China).
2.3 Instruments and equipment
The instruments and equipment included Biological Safety Cabinet (ThermoFisher, Waltham, MA, USA), hemocytometer (Yuhuan Qiujing Medical Instrument Factory, Yuhuan, China), electronic analytical balance (Shanghai Jingke Tianmei Trading Co., Ltd., Shanghai, China), dry bath incubator (K30, Hangzhou ALLSHENG Instruments Co.,Ltd., Hangzhou, China), refrigerated centrifuge (Eppendorf, Hamburg, Germany), Transwell 6-well plate (Corning, NY, USA), Costar Transwell 96-well plate (Corning, NY, USA), culture plate & culture dish (ThermoFisher, Waltham, MA, USA), NanoDrop spectrophotometer (ThermoFisher, Waltham, MA, USA), pipette (Eppendorf, Hamburg, Germany), high-pressure sterilization pot (Kagoshima Seisakusyo, Kagoshima, Kyushu, Japan), constant temperature incubator (ThermoFisher, Waltham, MA, USA), laboratory water bath (Changzhou Guohua Electric Appliance Co., Ltd., Changzhou, China), biological microscope (XINZHEN, Shanghai, China), horizontal shaker (Taicang Science and Education Equipment Factory, Suzhou, China), automatic microplate reader (Bio Rad, Hercules, CA, USA), Pro Flex PCR instrument (ThermoFisher, Waltham, MA, USA), fluorescent quantitative PCR instrument (ABI Corporation, Waltham, MA, USA), and electric thermostatic drying oven (Shanghai SENXIN Laboratory Instrument Co., Ltd., Shanghai, China).
2.4 Cell culture
THP-1 cells were cultured in RPMI1640 medium containing 10% serum (Gibco, Waltham, MA, USA), penicillin-streptomycin and 5% β-mercaptoethanol at 37 ℃ under 5% CO2 in an incubator. The half medium was refreshed every 2-3 days according to the condition of cell growth.
2.5 MTT assay for detecting the effects of Rhizoma Zedoariae extract and its active components on cell viability
THP-1 cells in a logarithmic phase (2 × 106/mL) were seeded in 96-well plates, and treated with Rhizoma Zedoariae extract, curcumin, germacrone, curcumol, curcumenol and curdione at different concentrations (0, 0.01, 0.1, 1, 3, 10, 30, 100, 300 μg/mL). The monomers with inhibitory effect on cell proliferation were screened, among which Rhizoma Zedoariae extract, curcumin and germacrone were more superior in suppressing cell proliferation. Later, THP-1 cells in a logarithmic phase (2 × 106/mL) were re-seeded in 96-well plates, and treated with Rhizoma Zedoariae extract (0.01, 0.1, 1, 10, 30, 100 μg/mL), curcumin (0.01, 0.1, 1, 10, 30, 100 μg/mL) and germacrone (0.01, 1, 10, 30, 100, 300 μg/mL), respectively, with the addition of 10 ng/mL G-CSF. Finally, the total volume of each well was 100 μL, and there were 4 parallel wells in each group. Normal group (only culture medium), control group (cells in culture medium) and control+10 ng/mL G-CSF group (cells in culture medium treated with 10 ng/mL G-CSF) were established. After 48 hours (h) of incubation, cells were reacted with 20 μL MTT reagent (5 mg/mL) for 4 h, centrifuged and then the supernatant was removed. 150 μL DMSO was added to cease the reaction. The optical density (OD) value of each well (450 nm) was detected using a microplate reader. Cell proliferation inhibition rate = 1 – (OD value of drug treatment group – OD value of normal group)/(OD value of control group – OD value of normal group) × 100%. The assay was repeated three times.
2.6 Quantitative real-time reverse transcription polymerase chain reaction (qRT-PCR) for quantification of IL-10, IL-6, JAK1, STAT3, NF-κB, TGF-β and ARG-1
Cells were grouped as those in MTT assay, and cultured in an incubator at 37 ℃ with 5% CO2 for 48 h. Total RNA was extracted as per the instructions of Total RNA Extractor, and reverse transcription of RNA into cDNA was implemented using cDNA reverse transcription kit. RT-PCR reaction system and condition were set. 20 μL PCR reagent was prepared in 0.5 mL PCR 8-strip tubes, gently mixed and centrifuged for 3 seconds (s). Temperature and cycle number were set according to primer property. Glyceraldehyde-3-phosphate dehydrogenase (GAPDH) acted as the internal control for calculating gene expression. Table 1 listed the primer sequences.
Table 1 Primer sequences.
| Gene | Sequence |
|---|---|
| ARG-1 | Forward: GTCTGTGGGAAAAGCAAGCG Reverse: CACCAGGCTGATTCTTCCGT |
| IL-10 | Forward: AAGTCCTGATGTCACTGCCC Reverse: AAGGTAAGGGGAGCCAGTTG |
| IL-6 | Forward: TGAACTCCTTCTCCACAAGCG Reverse: GCCTCTTTGCTGCTTTCACA |
| STAT3 | Forward: CTCTGCCGGAGAAACAGGAT Reverse: AGGTACCGTGTGTCAAGCTG |
| NF-κB | Forward: AGGCACATGGGATTAGCGAC Reverse: TGTAAGAGTTCCCCTCCGGT |
| JAK1 | Forward: AATTGCGGCAAGAAGGAAGC Reverse: TGCACCTGCTCAGACTTCTC |
| TGF-β | Forward: TCTCAGCGTGGTGAGGTATT Reverse: AGGCTTCATTTGTGGGAGCA |
| GAPDH | Forward: GTCTCCTCTGACTTCAACAGCG Reverse: ACCACCCTGTTGCTGTAGCCAA |
Note: Data analysis was completed based on standard curves and CT values automatically calculated by the software. The relative expressions of genes are calculated using the formula: Amplification factor 2-△△ct = 2-[(CT administration - CT model) - (CT administration internal reference - CT model internal reference)].
2.7 Statistical methods
Use GraphPad Prism 8.0 (Version 8.0; La Jolla, CA, USA) for statistical analysis. The data is represented by mean ± standard deviation. Multiple group comparisons are conducted using one-way ANOVA, and pairwise comparisons between groups are conducted using the least significant difference method. p < 0.05 indicates that the difference is statistically significant.
3 Results
3.1 The effects of Rhizoma Zedoariae extract and its active components on THP-1 cell viability
MTT assay results showed that after 48 h of treatment using Rhizoma Zedoariae extract and its active components, the proliferation of G-CSF (10 ng/mL)-induced cells was blocked by Rhizoma Zedoariae extract (0.1, 1, 10, 30, 100, 300 μg/mL), curcumin (0.01, 0.1, 1, 10, 30, 100 μg/mL), and germacrone (0.1, 1, 10, 30, 100, 300 μg/mL) (p < 0.01). Among them, Rhizoma Zedoariae extract at 100/300 μg/mL, curcumin at 30/100 μg/mL and germacrone at 100/300 μg/mL had better suppressive effects (p < 0.001), as described in Figures 1,2. Curcumol, curcumenol and curdione at different concentrations had little effect on the cell viability, and the cell survival rate of different concentration groups was above 70%, with insignificant difference among groups, so the data were not displayed in the Figure 1.
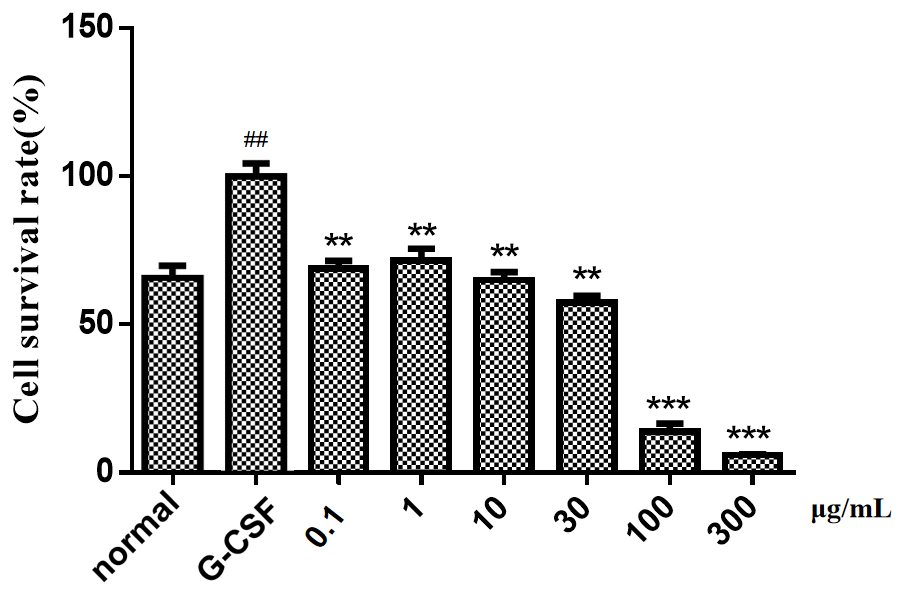
Figure 1 The effects of Rhizoma Zedoariae extract at different concentrations on G-CSF-induced THP-1 cell viability. MTT assay was performed to detect the changes of THP-1 cell viability in G-CSF group, normal group and Rhizoma Zedoariae extract group at 48 h. Compared with normal group, # p < 0.05, ## p < 0.01; Compared with G-CSF group, * p < 0.05, ** p < 0.01, *** p < 0.001.

Figure 2 The effects of curcumin and germacrone on G-CSF-induced THP-1 cell viability. MTT assay was conducted to test the changes of THP-1 cell viability in G-CSF group, normal group, curcumin group and germacrone group at 48 h. Compared with normal group, # p < 0.05, ## p < 0.01; Compared with G-CSF group, * p < 0.05, ** p < 0.01, *** p < 0.001.
3.2 The effects of Rhizoma Zedoariae extract, curcumin and germacrone on expressions of STAT3, ARG-1, IL-10 and JAK1 in THP-1 cells
The mRNA expression levels of TGF-β, IL-10, IL-6, JAK1, NF-κB, STAT3 and ARG-1 were quantified through qRT-PCR. After 24 h of G-CSF treatment, ARG-1 and IL-10 levels were elevated (p < 0.01), while STAT3 and JAK1 levels were reduced (p < 0.05) in THP-1 cells, as compared with normal cells. Compared with cells only subjected to treatment of G-CSF, Rhizoma Zedoariae extract at a high concentration of 300 μg/mL augmented ARG-1 and IL-10 mRNA levels (p < 0.01), but declined STAT3 and JAK1 mRNA levels in G-CSF-induced cells (p < 0.001) (Figure 3A-D). On the contrary, STAT3 mRNA levels were apparently increased by Rhizoma Zedoariae extract at a low concentration of 30 μg/mL in G-CSF-treated cells (p < 0.001) as compared with those in cells only treated with G-CSF, and the difference was not statistically significant in the concentration of 100 μg/mL (p > 0.05, Figure 3A-B). Rhizoma Zedoariae extract at a low concentration of 30 μg/mL elevated STAT3 level (p < 0.001) evidently but JAK1 level slightly. After treatment of Rhizoma Zedoariae extract at medium and low concentrations, IL-10 and ARG-1 mRNA levels were lessened and the differences were significantly except ARG-1 mRNA level under impacts of Rhizoma Zedoariae extract at a low concentration (p < 0.01, Figure 3C-D). Since curcumin and germacrone only fine-tuned the mRNA levels of TGF-β, IL-10, IL-6, JAK1, NF-κB, STAT3 and ARG-1, the relevant data were not exhibited in the manuscript.

Figure 3 The effects of Rhizoma Zedoariae extract on mRNA levels of STAT3 (A), JAK1 (B), IL-10 (C) and ARG-1 (D) in G-CSF-induced THP-1 cells. Compared with normal group, # p < 0.05, ## p < 0.01; Compared with G-CSF group, * p < 0.05, ** p < 0.01, *** p < 0.001.
4 Discussion
Chinese medicine has unique anti-tumor advantages, many Chinese medicine can reverse the mechanism of tumor in the immunosuppressive state, such as reversing the functions of T cells, enhancing the effect of dendritic cells (DCs), and regulating macrophages and natural killer cells [16]. Rhizoma Zedoariae, also called Lan Xin Jiang, Jiang Qi, and Hei Xin Jiang, is a smooth hairless perennial herb, and has the functions of dredging blood stasis, eliminating carbuncle and anti-tumor [17,18]. The effective constituent of Rhizoma Zedoariae, namely the volatile oil, has anti-viral, anti-inflammatory and anti-tumor properties. Among them, the anti-tumor property is achieved by directly destroying various cancer cells, and enhancing the specificity of the immune system. A previous study [19] revealed that curcumin enhances the sensitivity of breast cancer cells to chemotherapeutic agents. Besides, curcumin can trigger MDA-MB-231 cell apoptosis via regulating p21, Bax, Bcl-2 [20] and MMP-9 protein levels [21]. Clinical research data showed that germacrone can suppress proliferation of liver cancer, breast cancer and lung cancer cells [22,23]. Additionally, germacrone increases Caspase-3, Caspase-7, Caspase-9 and Bax expressions [24] and decreases Bcl-2 expression [23] to facilitate breast cancer cell apoptosis. In this study, Rhizoma Zedoariae extract, germacrone and curcumin exerted obvious inhibitory effects on cell viability in THP-1. Rhizoma Zedoariae extract in 100 μg/mL also decreased the ARG-1 and IL-10 mRNA levels in G-CSF induced THP-1, blocked the MDSCs-like differentiation of THP-1 cells. Furthermore, Rhizoma Zedoariae extract also significantly decreased the mRNA expressions of JAK1 and STAT3.
Splenomegaly occurs in over 50% of malignant tumors, can be caused by cell infiltration, splenic stasis, extramedullary hematopoiesis, histiocytic hyperplasia, fibrous tissue hyperplasia, and lipid dysbolism. Zhang et al. found that in dextran sulfate sodium (DSS)-induced inflammatory bowel disease (IBD) mice, the villi in the intestinal tissue of the mice are arranged in a disordered manner, curcumin can alleviate the above cell pathologies and enhance the functions of spleen and thymus possibly by regulating expressions of cytokines [25], such as TNF-α and IL-10. Curcuma kwangsiensis S. G. Lee et C. F. Liang, containing germacrone, curcumol, curdione, elemene and β-elemene, can effectively ameliorate syndromes of Chinese medicine in mice with hepatic fibrosis induced by blood stasis, increase body weight, mitigate liver distention, decrease liver and spleen indexes by mediating TGF-β1/Smads signaling pathway to downregulate TGF-β1, Smad2 and Smad3 protein and mRNA levels in liver tissues [26]. Reportedly [27-29], curcumin reduces MDSCs to repress tumor cell growth and migration. LIU Dan [30] demonstrated that curcumin suppresses the generation of ARG1 and ROS, promotes the expressions of mature myeloid cell surface markers (F4/80, MHCII, CDllc and CD80) in MDSCs, and restore the proportion of T cells in the spleen of tumor-bearing mice. Further, curcumin can not only reduces MDSCs, but also promote the maturation of MDSCs and attenuate their immunosuppressive function. It has been documented that curcumin exerts anti-tumor effects through modulating proliferation and function of MDSCs, and MDSCs are expected to act as therapeutic targets for cancers. Also, JianpiHuayu (invigorating spleen and removing blood stasis) decoction has been identified to attenuate the progression of H22 hepatocellular carcinoma grafted subcutaneously in mice by lessening the immunosuppressive function of MDSCs, boosting differentiation of MDSCs, and strengthening systemic anti-tumor immune response [31].
Tumor cells activate bone marrow through secreting relevant factors, including G-CSF, FLT3L, GM-CSF, TGF-β, IL-6 and IL-10, and intramedullary HSCs differentiate abnormally into IMCs [32,33], this is the main cause of splenomegaly in malignant tumors. G-CSF, a hematopoietic growth factor, has capable of facilitating the differentiation and maturation of neutrophils, which is commonly used to treat neutropenia following cancer chemotherapy. Breast cancer secretes G-CSF and other factors to induce the activation of bone marrow and generate massive IMCs that can differentiate into MDSCs [34], jointly impact the formation of tumor immunosuppressive environments. This study indicated that Rhizoma Zedoariae extract and its active components curcumin and germacrone inhibited the proliferation and differentiation of G-CSF-induced THP-1 cells. Meanwhile, qRT-PCR results confirmed that after 24 h of G-CSF treatment, ARG-1 and IL-10 levels were elevated, while STAT3 and JAK1 levels were diminished. The above data implied that Rhizoma Zedoariae extract can effectively treat splenomegaly, possibly through suppressing STAT3, IL-10, ARG1 and JAK1 mRNA expressions, and blocking G-CSF/STAT3 signal transduction to dampen the proliferation of IMCs in spleen, alleviate extramedullary hematopoiesis in spleen and inhibit the formation of an immunosuppressive environment in breast cancer.
Tumor and its stroma cells may secrete IL-6, IL-1β, TNF and S100A8/9 to activate STAT1, STAT3 [35] or NF-κB signaling pathway, and thus induce IMCs to differentiate into MDSCs in spleen. STAT3 is the key factor for T cell differentiation, which can be mediated by cell factors, such as IL-10 and IL-6. The confirmed activators of STAT3 are IL-6 family, interferon and G-CSF. The upstream JAK of STAT3, an important member of STAT family, is activated by cytokines or growth factors, then recruits STAT3 as a monomer in the cytoplasm, phosphorylates STAT molecule tyrosine, and transports them from the cytoplasm to the nucleus in dimer form, ultimately regulating transcription of target genes [36,37]. It has been documented that STAT3 suppresses the maturation of DCs, facilitates Treg accumulation and induces immunosuppressive microenvironment [38]. At the same time, STAT3 inhibits the neutrophile granulocytes- and NK cells-mediated killing effect and promotes the immune escape of tumor cells [39]. In our result, Rhizoma Zedoariae extract significantly decreased the mRNA expressions of JAK1 and STAT3, which indicated that the mechanism of Rhizoma Zedoariae’s anti-G-CSF induced MDSCs-like differentiation effect maybe related with blocking JAK1/STAT3 signal transduction in THP-1 cells.
Although this study has explored some new mechanisms of the anti breast cancer effect of Rhizoma Zedoariae, this study has not yet been verified in animal models. In this study, experiments on the STAT3 pathway and cell differentiation still need to be conducted using relevant inhibitors or agonists to carry out Western blot analysis, and further studies are needed to analyze whether the final differentiation state of myeloid cells has changed, as in cell models, using flow cytometry in animal models. Although it currently appears that the THP-1 cell model induced by G-CSF successfully indicates the inhibitory effect of Rhizoma Zedoariae on MDSC-like cell differentiation, further validation is needed for the role of this model in more candidate drugs.
5 Conclusion
Rhizoma Zedoariae extract, as well as its pure compounds germacrone and curcumin, effectively inhibited cell viability induced by G-CSF. Rhizoma Zedoariae extract also significantly suppresses the expression of IL-10 and ARG-1, shown inhibition effect to the G-CSF induced MDSCs-like differentiation of THP-1 cells, the mechanism maybe related with blocking JAK1/STAT3 signal transduction. This suggests the potential as therapeutic agents of Rhizoma Zedoariae extract in extramedullary hematopoiesis in spleen and the formation of an immunosuppressive environment in breast cancer and other chronic inflammatory diseases management.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
Author Contributions
F.H.: Conceptualization, Formal analysis, Writing - original draft; K.H.: Data curation, Methodology, Writing - review and editing. All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Consent to Participate
No ethical approval was required for this article.
Funding
Zhejiang Provincial Natural Science Foundation of China (LY19H280010).
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- Siegel RL, Miller KD, Wagle NS, et al. Cancer statistics, 2023. CA: A Cancer Journal for Clinicians 2023; 73(1): 1-32.
- Badwe R, Hawaldar R, Nair N, et al. Locoregional treatment versus no treatment of the primary tumour in metastatic breast cancer: an open-label randomised controlled trial. The Lancet Oncology 2015; 16(13): 1380-1388.
- Yang X, Tu Q, Zheng XQ, et al. Retrospective study of 577 patients with splenomegaly. The Practical Journal of Cancer 2010; 25(6): 632-635.
- Shou DW, Wen L, Song ZY, et al. Suppressive role of myeloid-derived suppressor cells (MDSCs) in the microenvironment of breast cancer and targeted immunotherapies. Oncotarget 2016; 7(39): 64505-64511.
- Alshetaiwi H, Pervolarakis N, Mcintyre LL, et al. Defining the emergence of myeloid-derived suppressor cells in breast cancer using single-cell transcriptomics. Science Immunology 2019; 5(44): eaay6017.
- Xu LL, Li LJ, Zhou W, et al. Research and application prospect of myeloid suppressor cells in tumor therapy. Clinical Focus 2017; 32(06): 545-548.
- Samantha S, Laura PT, Vera D, et al. Highlights on molecular mechanisms of MDSC-mediated immune suppression: paving the way for new working hypotheses. Immunological Investigations 2012; 41(6-7): 722-737.
- Gonda K, Shibata M, Ohtake T, et al. Myeloid-derived suppressor cells are increased and correlated with type 2 immune responses, malnutrition, inflammation, and poor prognosis in patients with breast cancer. Oncology Letters 2017; 14(2): 1766-1774.
- Solito S, Falisi E, Diaz-Montero CM, et al. A human promyelocytic-like population is responsible for the immune suppression mediated by myeloid-derived suppressor cells. Blood 2011, 118(8): 2254-2265.
- Serafini P, Borrello IV. Myeloid suppressor cells in cancer: recruitment, phenotype, properties, and mechanisms of immune suppression. Seminars in Cancer Biology 2006; 16(1): 53-65.
- Goh C, Narayanan S, Hahn YS. Myeloid-derived suppressor cells: the dark knight or the joker in viral infections? Immunological Reviews 2013; 255(1): 210-211.
- Diaz-Montero CM, Finke J, Montero AJ. Myeloid-derived suppressor cells in cancer: therapeutic, predictive, and prognostic implications. Seminars in Oncology 2014; 41(2): 174-184.
- Ni WT, Pan YH, Wang AY, et al. Research progress in inhibition of Chinese materia medica with breaking blood stasis and resolving mass on tumor metastasis. Chinese Herbal Medicine 2016; 47(24): 4472-4477.
- Li XQ, Lin ZH, Zhang B, et al. β-elemene sensitizes hepatocellular carcinoma cells to oxaliplatin by preventing oxaliplatin-induced degradation of copper transporter 1. Scientific Reports 2016; 6: 21010.
- Mao YE, Liu X, He K, et al. Xuanhusuo Powder inhibits myeloid-derived suppressor cells differentiation in the spleen of breast cancer mice by down-regulating G-CSF. Journal of Zhejiang University (Medical Science) 2023; 52(01): 88-100.
- He BQ, Guo WQ, Shi RZ, et al. Ruyong Formula improves thymus function of CUMS-stimulated breast cancer mice. Journal of Ethnopharmacology 2023; 319: 117164.
- Tang DC, Zang WH, Feng HH. Influences of medicated serum of different cultivars of Ezhu (Rhizoma Zedoariae) on proliferation, apoptosis and nucleo-cytoplasmic ratio in human gastric carcinoma cells BGC823. Journal of Beijing University of Traditional Chinese Medicine 2013; 36(04): 254-257+267+289.
- Tang X, Han FJ, Li W, et al. Research on the effect of Curcumol in JAK2/STAT3 signaling pathway in human ovarian cancer cell line SKOV3. Chinese Journal of Clinical Obstetrics and Gynecology 2013; 14(01): 43-46.
- Li HH, Ren WL, Xu CW, et al. Study on the effect of curcumin on triple negative breast cancer cells drug sensitivity and exploring the mechanism. Journal of Modern Oncology 2019; 27(23): 4139-4144.
- Chiu TL, Su CC. Curcumin inhibits proliferation and migration by increasing the Bax to Bcl-2 ratio and decreasing NF-kappaBp65 expression in breast cancer MDA-MB-231 cells. International Journal of Molecular Medicine 2009; 23(4): 469-475.
- Qi XG, Han X, Hao JZ, et al. Mechanism of curcumin inhibiting proliferation and invasion of breast cancer cells. Chinese Journal of Cancer Prevention and Treatment 2018; 25(17): 1211-1216.
- Chen QF, Wang G, Li ZF, et al. Research progress of germacrone pharmacological effects. Chinese Archives of Traditional Chinese Medicine 2017; 35(09): 2312-2315.
- Liu YY, Zheng Q, Fang B, et al. Germacrone induces apoptosis in human hepatoma HepG2 cells through inhibition of the JAK2/STAT3 signalling pathway. Journal of Huazhong University of Science and Technology - Medical Science 2013; 33(03): 339-345.
- Zhong ZF, Chen XP, Tan W, et al. Germacrone inhibits the proliferation of breast cancer cell lines by inducing cell cycle arrest and promoting apoptosis. European Journal of Pharmacology 2011; 667(1-3): 50-55.
- Zhang CH, Li ZY, Luo XF, et al. Mechanism of curcumin on injury repair in mice with inflammatory bowel disease induced bydextran sulfate sodium. Heilongjiang Animal Science and Veterinary Medicine 2019; 23: 15-17+168.
- Liu LL. Effect of curcuma kwangsiensis S. G. Lee et C. F. Liang on TGF-β1/Smads signaling pathway in mice with hepatic fibrosis induced by blood stasis. Journal of Guangxi University of Chinese Medicine 2019.
- Lu Y, Miao L, Wang YH, et al. Curcumin Micelles Remodel Tumor Microenvironment and Enhance Vaccine Activity in an Advanced Melanoma Model. Molecular Therapy 2016; 24(2): 364-374.
- Singh M, Ramos I, Asafu-Adjel D, et al. Curcumin improves the therapeutic efficacy of a Listeria(at)-Mage-b vaccine in correlation with improved T-cell responses in blood of a triple-negative breast cancer model 4T1. Cancer Medicine 2013; 2(4): 571-582.
- Tu SP, Jin HY, Si JD, et al. Curcumin lnduces the Differentiation of Myeloid-Derived Suppressor Cells and Inhibits Their Interaction with Cancer Cells and Related Tumor Growth. Cancer Prevention Research 2012; 5(2): 205-215.
- Liu D. The novel α-glucan and curcumin ameliorate inflammatory diseases through modulating the expansion and immunosuppressive function of MDSCs. Nanjing University: Nanjing, China, 2016.
- Xie YJ, Zhang Y, Wei XH, et al. JianpiHuayu Decoction Attenuates the Immunosuppressive Status of H22 Hepatocellular Carcinoma-Bearing Mice: By Targeting Myeloid-Derived Suppressor Cells. Frontiers in Pharmacology 2020; 11: 16.
- Giles A, Reid C, Evans J, et al. Activation of hematopoietic stem/progenitor cells promotes immunosuppression within the pre-metastatic niche. Cancer Research 2016; 76(6): 1335-1347.
- Atretkhany K, Nosenko M, Gogoleva V, et al. TNF Neutralization Results in the Delay of Transplantable Tumor Growth and Reduced MDSC Accumulation Frontiers in Immunology. Frontiers in Immunology 2016; 7(8): 147-158.
- He K, Liu X, Hoffman RD, et al. G-CSF/GM-CSF-induced hematopoietic dysregulation in the progression of solid tumors. FEBS Open Bio 2022; 12(7): 1268-1285.
- Pan T, Liu YF, Zhong LM, et al. Myeloid-derived suppressor cells are essential for maintaining feto-maternal immunotolerance via STAT3 signaling in mice. Journal of Leukocyte Biology 2016; 100(3): 499-511.
- Yang Q, Li X, Chen H, et al. IRF7 regulates the development of granulocytic myeloid-derived suppressor cells through S100A9 transrepression in cancer. Oncogene 2017; 36(21): 2969-2980.
- Zhu RL, Zhi YK, Yi L, et al. Sinomenine regulates CD14/TLR4, JAK2/STAT3 pathway and calcium signal via α7nAChR to inhibit inflammation in LPS-stimulated macrophages. Immunopharmacology and Immunotoxicology 2019; 41(1): 172-177.
- Cocchiola R, Rubini E, Altieri F, et al. STAT3 post-translational modifications drive cellular signaling pathways in prostate cancer cells. International Journal of Molecular Sciences 2019; 20(8): E1815.
- Hua Y, Marcin K, Drew P. Crosstalk between cancer and immune cells: role of STAT3 in the tumour microenvironment. Nature Reviews Immunology 2007; 7(1): 41-51.