Main Text
Graphic Abstract
Introduction
Obesity is a kind of heterogeneous syndrome. The occurrence of obesity is associated with a variety of metabolic syndrome, including diabetes, liver steatosis, cardiovascular disease, etc [1,2]. According to the latest data from the Report on the Nutrition and Chronic Diseases Status of Chinese Residents (2020), more than half of Chinese adults are overweight or obese, of which 34.3% are overweight and 16.4% are obese (≥18 years of age) [3]. In this nationwide survey, the proportion exceeded 50% for the first time. Obesity is closely related to the development of three high disease (hypertension, hyperlipidemia, hyperglycemia), atherosclerosis and other diseases, as well as the decline of heart function, which seriously threatens human health.
According to the pathogenesis and clinical development process, obesity can be classified as primary obesity, also known as simple obesity; And secondary obesity, also known as symptomatic obesity [4,5]. Among them, simple obesity often arises from an overconsumption of calories. For example, a lack of dietary regulation and the consumption of high-fat or calorie-dense foods can lead to the accumulation of adipose tissue, while excessive eating may also contribute to its development. However, if simple obesity is not addressed with due seriousness, it may progress into secondary obesity. Clinical research primarily focused on secondary obesity which is predominantly related to hormone imbalance. According to literature research, this paper categorized obesity into hyperinsulinemic obesity [6], Cushing syndrome obesity [7], hypothalamic obesity [8], hypothyroid obesity [9], and polycystic ovary syndrome based on their respective pathogenesis and pathogenic sites [10]. Secondary obesity is mainly caused by abnormal endocrine metabolism. Hyperinsulinemic obesity results from excessive insulin secretion in terms of hormone metabolism [11], while Cushing's syndrome-related obesity stems from overproduction of glucocorticoids [12]. Hypothyroidism-induced obesity is linked to the disorder of glucose metabolism [13], and hypothalamic obesity arises due to damage to the satiety and feeding centers, leading to an abundance of glucose-sensing neurons and various neuropeptide receptors in the satiety center [14,15]. These neurons are involved in the regulation of energy balance and the metabolism of substances such as melanin and glutamic acid produced by the central nervous system [16,17]. Additionally, polycystic ovary syndrome is characterized by excessive androgen activity or hyperandrogenism [18,19].
The classification, symptomatology, and underlying mechanisms of obesity are delineated in Table 1. Although diet pills have certain effects on weight loss, prolonged use may result in adverse side effects. Over the past few decades, numerous weight-loss pills have been withdrawn from the market due to their cardiotoxicity. In recent years, although many diet pills have played a certain role in the short-term treatment of obesity, they also exert significant impacts on patients' physical and mental health. For instance, Orlistat Capsules, as a typical weight loss product in the market, prevent fat absorption by inhibiting gut enzymes that absorb fat [20,21]. However, this has been confirmed to cause adverse reactions such as diarrhea, abdominal pain and liver toxicity. A clinical study assessing drug has demonstrated that weight-loss medications, such as Lorcaserin, may elevate the risk of cancer [22,23].
Table 1 Types of obesity are classified based on various neuroendocrine and metabolic disorders.
| Classification | Characteristics | Mechanism | |
|---|---|---|---|
| Isolated obesity | Not related to endocrine system diseases | The body fat is evenly distributed | Excessive-intake;no organic disease |
| Secondary obesity | Hyperinsulinemic obesity | Abominal obesity | Iinsulin overproduction |
| Cushing syndrome obesity | Centripetal obesity | Excess glucocorticoids secretion | |
| Hypothalamic obesity | A strong appetite | Disorder of melanin-concentrating hormone metabolism | |
| Hypothyroid obesity | Reduced systemic metabolism | Disorder of glucose metabolism | |
| Polycystic ovary syndrome | Abominal obesity | Excessive androgen secretion | |
In China, there has been a long-standing belief that food and medicine share the same origin [24,25]. This implies that many foods possess dual properties of both nourishment and healing. The homologous function of medicine and food aligns with the modern concept of nutritional immunology, encompassing four key aspects: 1) balancing the human body and regulating endocrine glands to promote normal endocrine function; 2) providing a natural cleansing effect; 3) supplying vitamins, minerals, and other essential nutrients; and 4) furnishing the immune system with vital nourishment.
According to the principles of traditional Chinese medicine, lotus leaves are characterized as having a flat nature and bitter taste, and they affect the liver, spleen, stomach, and heart meridians. Its effects include clearing summer-heat and dampness, promoting lucid yang energy, cooling blood, and stopping bleeding. In dietary therapies such as winter melon lotus leaf tea, lotus leaf purple cabbage soup, lotus leaf chicken and lotus leaf rice, the small molecules of lotus leaf can effectively penetrate into the food to achieve its desired effect of clearing heat and reducing fat. Lotus leaves are widely distributed in ponds, lakes, and paddy fields throughout North and South China. The phrase "out of the mud and not dyed" is commonly used to praise the lotus, but few know that its true essence lies within the lotus leaf. According to historical records, lotus leaves have been utilized for medicinal purposes in China for over 2,000 years. During the Qin and Han dynasties, they were first employed as a tonic medicine. Meanwhile, lotus leaf is also considered a weight loss aid among the populace. The lipid-lowering effects of lotus leaves have been documented in numerous ancient texts, such as the Compendium of Materia Medica, which states that "lotus leaves generate Yang qi, reduce fat and promote weight loss." In Qing Dynasty's "Materia Medica Truth-seeking" it is noted that "consuming more lotus leaves will lead to slimming and toning," indicating that increased consumption of lotus leaves can result in weight reduction.
The lotus leaf and its derivatives have been documented in contemporary scientific research for their potential to exert anti-obesity effects [26]. Among them, the study revealed that the fermentation supernatant of lotus leaf inhibited adipogenesis in 3T3-L1 preadipocytes induced by a high-fat diet in obese rats, thereby exerting an inhibitory effect on obesity [27]. Additionally, it has been reported that lotus leaf extract exhibits anti-obesity effects in obese mice fed a high-fat diet [28]. Furthermore, the study also investigated the impact and mechanism of ethanol extract from lotus leaf on intestinal microbiome and obesity in rats with a high-fat diet [29].
Therefore, in order to facilitate more profound and comprehensive investigation and exploration into the anti-obesity potential of lotus leaf, as well as to further enhance its clinical applicability, it is imperative to conduct a meticulous review and synthesis of its chemical composition and pharmacological activity, thereby establishing a solid foundation for subsequent in-depth research and practical implementation.
The correlation between identified monomeric drug targets in lotus leaves and their potential role in treating obesity
We employed the Traditional Chinese Medicine Systems Pharmacology (TCMSP) database and analysis platform to explore the chemical composition of lotus leaf [30,31]. The database revealed that 105 active ingredients have been isolated and identified in the previous researches, and subsequently, we conducted Gene Ontology (GO) analysis on the reported target genes of these compounds. The screening threshold was set at false discovery rate (FDR)<0.05, and the analysis revealed that the top 20 biological processes were primarily associated with responses to oxygen levels, carboxylic acid and organic acid biosynthesis, as well as lipid localization. The results of the GO analysis are presented in Figure 1A.
Moreover, we have identified 52 compounds out of the 105 active ingredients that exhibit an oral bioavailability (OB) greater than 20% according to the TCMSP database. We then determined the pharmacodynamic targets of these monomers and utilized disease-related databases such as GeneCards, DisGeNet, DISEASE, and OMIM to extract genes associated with obesity [32]. The extracted disease-related genes were integrated with drug targets for further analysis. The analytical procedure is illustrated in Figure 1B.
The monomer component of lotus leaf (OB>20%, based on the screening criteria established in previous research [33]) targets are 80% related to obesity, as shown in Figure 1C, indicating its potential anti-obesity function. Further investigation into the underlying mechanisms and development of health products holds significant research value.
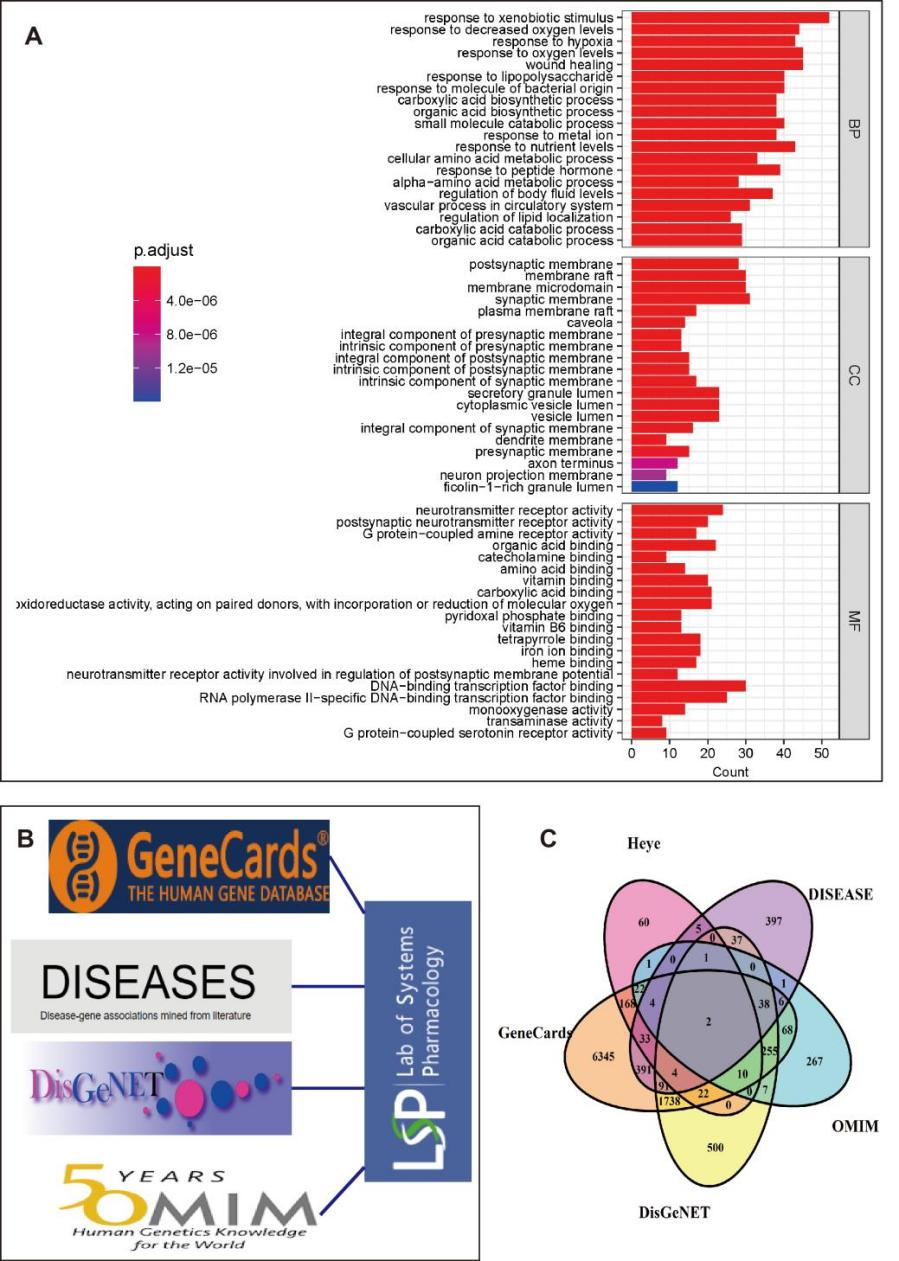
Figure 1 Integration of disease databases was conducted to analyze the correlation between drug targets of lotus leaf components with oral availability greater than 20% and obesity. A. Performing GO-analysis to predict the molecular functions of putative targets of lotus leaf components. B. The utilized databases for integrated analysis were presented. C. The Venn diagram demonstrated the results obtained from panel A.
The monomeric constituents of lotus leaf associated with obesity have been documented
Based on the ranking of oral availability of monomer components in lotus leaves, we conducted a literature review and summarized the reported active ingredients for anti-obesity related diseases with an OB greater than 20%. Furthermore, based on the classification of different types of compounds, we present our findings as follows:
Flavonoids
Flavonoids, existing in the form of flavonoid glycosides and aglycones, are a significant class of secondary metabolites in plants. They can be found in various plant species such as fruits, vegetables, legumes, and tea. Research has shown that low-density lipoprotein is detrimental to human health and may lead to coronary heart disease [34,35]. Flavonoids have been shown to inhibit the production of harmful low-density lipoprotein and reduce the formation of blood clots, leading to a lower mortality rate from coronary heart disease in individuals with high flavonoid intake [36-38]. Additionally, research suggests that the flavonoid components may effectively reduce the risk of cancers, cardiovascular diseases, gynecological disorders, metabolic disorders, musculoskeletal conditions, endocrine dysfunctions, neurological disorders, and renal complications, particularly in perimenopausal women [39].
The ingredients of reported flavonoids associated with obesity in lotus leaf are presented in Table 2.
Table 2 Main flavonoids with Anti-obesity activity in Folium Nelumbinis.
| Chemical structure | Compound’s name | Oral availability (%) | Tested animals | Molecular mechanism |
|---|---|---|---|---|
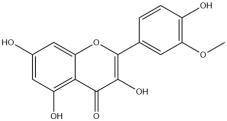 | Isorhamnetin | 49.5 | Caenorhabditis elegans; High-fat diet-induced mice | NHR-49/PPARα-dependent pathway;Activation of the AMPK-GLUT4 pathway |
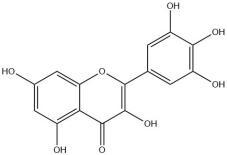 | Myricetin | 13.75 | High choline-fed mice; rats | Activation of PI3K/AKT signaling pathway |
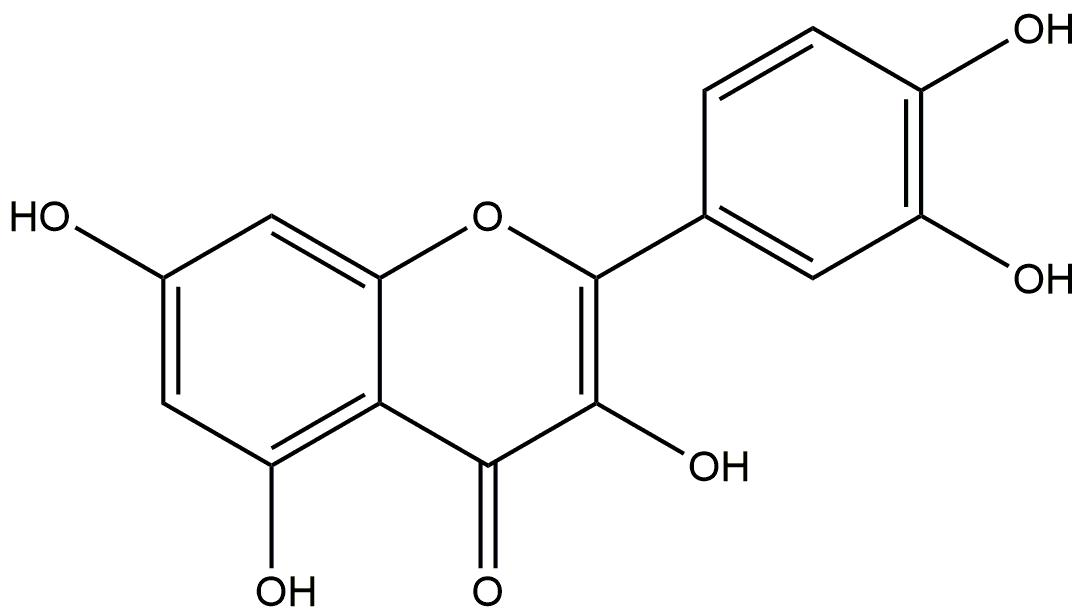 | Quercetin | 46.43 | Wistar Rats with Diet-Induced Obesity | Inhibiting the pro-inflammatory molecules |
Isorhamnetin
Isorhamnetin is a flavonoid compound found in fruits and herbs, specifically as one of the components in lotus leaves. With an oral bioavailability of 49.6%, it has been shown to possess various functions such as reducing blood pressure, lowering blood lipids, decreasing blood viscosity, enhancing vascular elasticity, and exhibiting anti-atherosclerotic properties [40,41]. It can be considered a potent agent against cardiovascular and cerebrovascular diseases.
The research has demonstrated that isohamnetin can upregulate the NHR-49/PPARα pathway, which plays a crucial role in enhancing fat oxidation and reducing body fat [36,42,43]. Moreover, isorhamnetin has been found to exert its anti-inflammatory and glucose-lowering effects by modulating AMPK-GLUT4 signaling pathway in a high-fat drink induced type 2 diabetes mouse model [43,44].
Myricetin
Myricetin, a bioflavonoid primarily extracted from bayberry, has an oral availability of 13.75%. Numerous studies have demonstrated its various cardiovascular pharmacological effects such as anti-thrombosis, anti-myocardial ischemia, and improvement of microcirculation [45,46]. In addition, it exhibits a diverse range of pharmacological effects such as hypoglycemic, antioxidant, hepatoprotective and choleretic activities [47].
Furthermore, in the LDL oxidation experiment, myricetin was found to inhibit LDL oxidation [48]. Myricetin not only possesses pharmacological properties including anti-inflammatory, antitumor, antimutagenic and cariostatic effects as well as antioxidative and free radical scavenging activities but also exerts its maximum effect through glucose inhibition [49,50]. In vitro studies have demonstrated that myricetin reduces blood glucose levels by increasing myocyte uptake and glycogen synthesis [51,52]. The anti-obesity mechanism of Myricetin exerts has been reported to involve the upregulation of SIRT3 in adipose tissue, thereby exerting its anti-obesity effects [53]. However, the precise mechanism of action remains to be elucidated. In summary, myricetin has been shown to inhibit glucose uptake into cultured rat adipocytes.
Quercetin
Quercetin, contained in lotus leaves, exhibited an oral availability of 46.43%. In obese Wistar Rats fed with a high-fat diet and treated with quercetin, total RNA was extracted from the epididymal adipose tissue. The results showed that rats treated with quercetin lost 31% of their body weight compared to the obese control group [54]. Quercetin was also found to suppress the expression of pro-inflammatory cytokines including IL-6, IL-1β, IL-18, Lep, HiF-1ɑ and NF-kB while leaving Socs1 and Socs3 unaffected [55]. These findings suggest that quercetin may modulate the obesity-associated inflammatory response by downregulating pro-inflammatory gene expression and altering signaling pathways.
Organic acids
Organic acids are nutritional components that exist in plants and are widely distributed in the fruits, leaves, and roots of Chinese herbs. Lotus leaves contain a plethora of organic acids. Natural organic acids, such as malic acid, can enhance human metabolism and effectively prevent obesity while playing a role in weight loss [56]. Catechuic acid has pharmacological effects including anti-tumor and anti-oxidation properties [57,58]. The abundance of organic acids found in lotus leaf confirms its efficacy in treating obesity to some extent.
Myristic acid
Myristic acid is a saturated fatty acid with therapeutic potential in traditional Chinese medicine, where it is used to promote digestion [59]. As a warm drug, its oral bioavailability has been determined to be 21.18%. Recent studies have demonstrated the efficacy of myristic acid in managing type-2 diabetes [60]. The Nagoya-Shibata-Yasuda (NSY) mice were selected as the model for Type-2 diabetes in this study, and were treated with 300mg/kg myristic acid or control agents through oral administration every other day [61]. Glucose and insulin tolerance tests were conducted at different time points, revealing that NSY male mice exhibited higher glucose tolerance levels than the control group, while their insulin-responsive blood sugar levels were lower. Meanwhile, myristic acid also decreased the body weight gain of NSY mice, indicating a strong correlation between myristic acid and insulin-obesity. However, several studies have demonstrated that myristic acid, a type of saturated fatty acid (SFA), is considered detrimental to human health as it can exacerbate adipose inflammation and systemic insulin resistance induced by a high-fat diet [62]. Therefore, further research is necessary to fully comprehend the underlying molecular mechanisms involved.
Palmitic acid
Palmitic acid, a saturated fatty acid found in various plants and animals, has an oral bioavailability of 19.31%. Studies have demonstrated that a diet high in Palmitic acid can induce elevated insulin levels in rats [63]. Furthermore, it has been discovered that palmitic acid down-regulates miR-221 expression levels, which reduces glucose uptake by HepG2 cells and inhibits the PI3K/AKT signaling pathway through binding with PI3K mRNA. Ultimately, this may prevent and treat insulin resistance induced by Palmitic acid.
Citric acid
Citric acid is a natural antiseptic and food additive with an oral bioavailability of 56.22%. Appropriate consumption of citric acid can stimulate appetite, enhance gastric juice secretion, alleviate indigestion symptoms, and improve body metabolism [64,65]. Recent experimental research has demonstrated that a mixture containing citric acid can effectively treat and prevent non-insulin dependent diabetes in mice [66]. Furthermore, it has been reported that soluble dietary fiber polyglucose [67], which also contains citric acid, may be utilized to manage and prevent obesity in HFD-fed mice. These studies have exhibited the capacity of citric acid to reduce weight levels, fasting blood glucose levels, and adipose tissue accumulation. Overall, these findings suggest that citric acid exerts a significant impact on lipid metabolism to some extent, and further investigation is warranted to elucidate the underlying molecular mechanisms.
Gallic acid
Gallic acid is widely distributed in various plants, such as swamp mahogany and dogwood. It can also be naturally extracted from sources like pomegranate, tea leaves, lotus leaves, among others. Modern pharmacological research has revealed that gallic acid exhibits multiple functions including anti-inflammatory, antioxidant, and antibacterial properties [68,69]. Recent research has demonstrated that intraperitoneal administration of gallic acid in mice with diet-induced obesity can significantly enhance their glucose tolerance and lipid metabolism [70]. Moreover, gallic acid also exhibits a certain effect on ameliorating fatty liver degeneration in mice [71]. These findings suggest that lotus leaf-derived gallic acid may hold potential therapeutic benefits for high blood glucose-dependent obesity.
The ingredients of reported Organic acids associated with obesity in lotus leaf are presented in Table 3.
Table 3 Main Organic acids with Anti-obesity activity in Folium Nelumbinis.
| Chemical structure | Compound’s name | Oral availability (%) | Tested animals | Molecular mechanism |
|---|---|---|---|---|
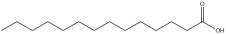 | Myristic acid | 21.18 | Nagoya-Shibata-Yasuda mouse model | NA |
 | Palmitic acid | 19.31 | Sirt3-MKO mice | PI3K/AKT/GLUT4 Pathway |
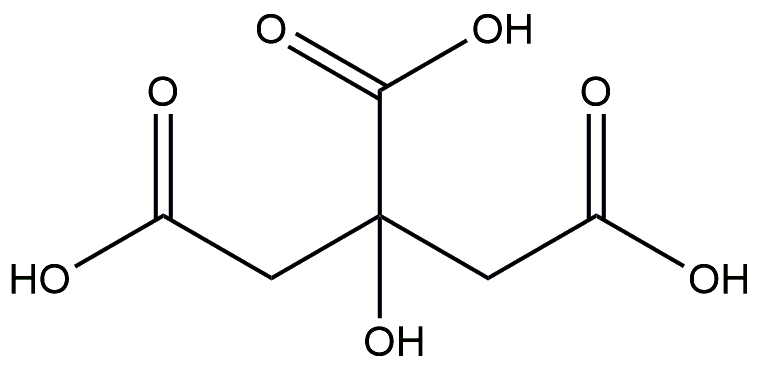 | Citric acid | 56.22 | HFD-fed mice | NA |
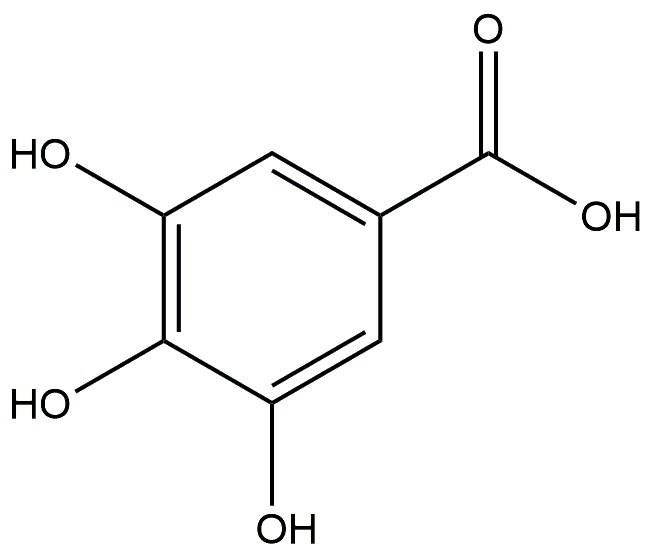 | Gallic acid | 31.69 | HFD-fed mice | NA |
Alkaloids
The lotus leaf contains a variety of alkaloids, including nuciferin, papaverine, methylaconitine, and malic acid as well as some tannins. The reduction of lipid levels and blood pressure has been documented in numerous studies.
Nuciferine
Nuciferine, the primary lipid-lowering active compound in lotus leaves, which has been reported that an active ingredient derived from lotus leaf has the potential to be used as a treatment for obesity and obesity-related diseases [72], exhibits an oral bioavailability of 34.43%.
The previous study has demonstrated that Nuciferine modulates the gut microbiota and confers protection against obesity in rats fed a high-fat diet [42]. Its anti-inflammatory properties have been attributed to its ability to inhibit p38 MAPK/ATF2 signaling pathway stimulated by Lipopolysaccharide [73].
Armepavine
Lotus leaves are abundant in Armepavine, exhibiting an oral utilization rate of 69.31%. Prior research has demonstrated that Armepavine not only possesses anti-inflammatory properties on human peripheral blood Monocyte and immunosuppressive effects on T lymphocytes and lupus nephritis mice, but also exhibits hypolipidemic effects [74,75]. In articles related to these studies, researches employed total cholesterol (TC) and total triglycerides (TG) as evaluation parameters, utilizing a hyperlipidemia model to assess the efficacy of Jiang-zhi-ning, a weight loss product containing an effective extract. The results indicated that Armepavine was one of five active components found in Jiang-zhi-ning through an "active contribution study," which preliminarily demonstrated its ability to reduce blood lipids [74,76].
The ingredients of reported alkaloids associated with obesity in lotus leaf are presented in Table 4.
Table 4 Main Alkaloids with Anti-obesity activity in Folium Nelumbinis.
| Chemical structure | Compound’s name | Oral availability (%) | Tested animals | Molecular mechanism |
|---|---|---|---|---|
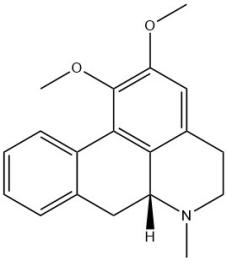 | Nuciferine | 34.43 | NA | Inhibiting the NF-κB and p38 MAPK/ATF2 signaling pathways |
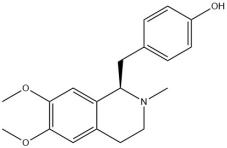 | Armepavine | 69.31 | NA | NA |
Discussion
Obesity is a type of non-communicable disease that has become the leading cause of mortality, posing a significant threat to human health. In terms of physical consequences, obesity can lead to complications such as hyperlipidemia, coronary heart disease, cardiomyopathy, hematangiosis and even cancer. In terms of psychology, adolescents who suffer from obesity-induced physical limitations or difficulties are vulnerable to bullying and aggression from their peers, leading to social disengagement and feelings of self-deprecation. In severe cases, this may even result in the development of autism spectrum disorder. Even for adults, the dilemma caused by obesity in terms of job opportunities loss, family and economy can lead to psychological distress, ultimately resulting in resentment towards society and posing a danger to both oneself and others.
Based on the aforementioned negative effects of obesity, weight loss is imperative for all individuals and a variety of weight loss medications have been developed in the past. These weight loss medications contain fenfluramine, a catecholamine, as well as naltrexone, an opioid antagonist, and serotonin receptor agonists that aim to promote weight loss by activating metabolism, suppressing hyperactive appetite, and reducing fat absorption in the gastrointestinal tract. While drugs that have appetite suppression functions are commonly used to treat obesity caused by bulimia, their significant impact on vice function means they should not be prescribed for the general population.For instance, the administration of fenfluramine may readily induce insomnia, thirst, and constipation, while that of naltrexone may easily lead to nausea, vomiting, and headache.
The use of drugs and food in traditional Chinese medicine has a long history, as recorded in Huangdi's Inner Classic [77,78]: "Food is used to satisfy hunger, while medicine is used to treat illness." In the earliest pharmacology monograph, Shennong's Herbal Classic, many common foods were classified as top-grade medicines, indicating that there is no clear boundary between food and medicine. A substance with the same origin as medicine and food possesses the essence of edible Chinese medicinal materials, exhibiting dual functionality in both medicine and cuisine. Its primary function is that of a food rather than a drug.They are primarily reinforcing drugs that serve the functions of recuperation, rehabilitation, and healthcare. These drugs are commonly used in healthcare as part of food therapy, dietary supplementation, and herbal cuisine. According to traditional Chinese medicine, obesity is closely related to one's innate constitution and dietary habits. Patients with this condition tend to be sedentary and averse to physical exercise. The therapy methods utilize the principles of tonifying deficiencies and draining excesses, as well as promoting qi and eliminating phlegm, dissolving water, eliminating fu-organs, removing blood stasis, etc., based on syndrome differentiation and treatment [79,80]. Ultimately, these methods regulate the deficiency of Zang-fu Qi blood Yin and Yang. As previously mentioned in both theoretical and practical experiences of Chinese medicine, the utilization of medicinal and dietary sources from the same origin is recommended for preventing and treating obesity.
The lotus leaf possesses primary functions such as heat-clearing, promoting defecation, strengthening the spleen, and enhancing Yang energy. Furthermore, it has a history of thousands of years in China as an effective aid for weight loss, representing the harmonious integration of medicine and food. In this study, we utilized multiple databases to integrate and analyze the targets of lotus leaf associated with obesity-related targets, ensuring that approximately 80% of the identified targets in lotus leaf are related to obesity. This finding confirms the traditional usage experience of lotus leaf for weight loss in China over many years. Next, we conducted an analysis and ranking of the active components in lotus leaf based on their oral bioavailability, and combined this with a thorough literature search to summarize the reported active components with anti-obesity properties. Through our research on the aforementioned components, we have discovered numerous compounds with superior anti-obesity properties. For instance, certain alkaloids possess a clear anti-obesity function, while flavonoids and organic acids have also been reported to exhibit such effects. However, the molecular mechanisms underlying these active weight loss components remain unclear, and there are still numerous bioactive components that have yet to be explored, as is the case with other Chinese medicines [81,82]. The field of Chinese herbal medicine offers a wide range of research opportunities, providing valuable inspiration for future studies. Thorough investigation will be necessary for future research.
Conclusion and prospect
The lotus leaf, a renowned traditional Chinese medicine, has been extensively researched in recent decades due to its multifaceted biological activities, particularly its anti-obesity potential. It contains several exceptional monomers and extracts that demonstrate significant anti-obesity effects. However, the majority of existing research on its mechanism of action lacks systematic approaches such as multi-omics investigations, impeding its widespread application and promotion in this field. The present paper provides the first review of the anti-obesity effect of lotus leaf monomer component, which holds significant reference value for further exploration into healthy dietary practices.
Back Matter
Acknowledgments
We express our gratitude to Guaikai Li for providing us with the photograph of lotus in Jiaoshan, Zhen Jiang, Jiangsu province of China.
Conflicts of Interest
The authors declare that they have no competing interests.
Author Contributions
Yuwei Liu: Conceptualization, Methodology, Lei Chen, Xinyi Zhang:Writing - Original draft and Editing. Yuwei Liu and Wei Zhang, Writing - Reviewing and Editing. Yuwei Liu and Wei Zhang: Supervision.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee, and the patients were informed and consented.
Funding
This work was supported financially by the National Natural Science Foundation of China (82101630). We thank Shani Xiangyi Wang, from Shanghai High School International Division, for checking the grammar of this manuscript.
Availability of Data and Materials
All data generated or analysed during this study are included in this published article.
Supplementary Materials
Not applicable.
References
- James A, Wang K, Wang Y. Therapeutic Activity of Green Tea Epigallocatechin-3-Gallate on Metabolic Diseases and Non-Alcoholic Fatty Liver Diseases: The Current Updates. Nutrients 2023; 15(13).
- Tutunchi H, Arefhosseini S, Nomi-Golzar S, et al. Effects of Hydroxycitric Acid Supplementation on Body Composition, Obesity Indices, Appetite, Leptin, and Adiponectin of Women with NAFLD on a Calorie-Restricted Diet. International Journal of Clinical Practice 2023; 2023: 6492478.
- Report on the Nutrition and Chronic Disease Status of Chinese Residents. SCIO Press Conferences: Beijing, China, 2023.
- Khadilkar V, Shah N. Evaluation of Children and Adolescents with Obesity. Indian Journal of Pediatrics 2021; 88(12): 1214-1221.
- Obita G, Alkhatib A. Effectiveness of Lifestyle Nutrition and Physical Activity Interventions for Childhood Obesity and Associated Comorbidities among Children from Minority Ethnic Groups: A Systematic Review and Meta-Analysis. Nutrients 2023; 15(11): 2524.
- Alemón-Medina R, Chávez-Pacheco JL, Rivera-Espinosa L, et al. Extemporaneous Formulations of Metformin for Pediatric Endocrinology: Physicochemical Integrity, Cytotoxicity of Sweeteners, and Quantitation of Plasma Levels. Clinical Therapeutics 2015; 37(8): 1689-1702.
- Lee WH, Larsson SC, Wood A, et al. Genetically predicted plasma cortisol and common chronic diseases: A Mendelian randomization study. Clinical Endocrinology 2023; 100(3): 238-244.
- Van Schaik J, Schouten-van Meeteren AYN, Vos-Kerkhof E, et al. Treatment and outcome of the Dutch Childhood Craniopharyngioma Cohort study: first results after centralization of care. Neuro-Oncology 2023; 25(12): 2250-2261.
- Dehdari Ebrahimi N, Sadeghi A, Ala M, et al. Protective effects of melatonin against oxidative stress induced by metabolic disorders in the male reproductive system: a systematic review and meta-analysis of rodent models. Frontiers in Endocrinology 2023; 14: 1202560.
- Shen C, Jiang Y, Lin J, et al. Purinergic receptor P2 × 7 activates NOX2/JNK signaling to participate in granulosa cell inflammation and apoptosis in polycystic ovary syndrome. Journal of Bioenergetics and Biomembranes 2023; 55(4): 313-322.
- Saniotis A. Is hyperinsulinemia a possible clinical explanation underlying the myth of Erysichthon? Acta Diabetologica 2023; 60(9): 1279-1282.
- Saad-Omer SM, Kinaan M, Matos M, et al. Exogenous Cushing Syndrome and Hip Fracture Due to Over-the-Counter Supplement (Artri King). Cureus 2023; 15(7): e41278.
- Yan Y, Niu Z, Sun C, et al. Hepatic thyroid hormone signalling modulates glucose homeostasis through the regulation of GLP-1 production via bile acid-mediated FXR antagonism. Nature Communications 2022; 13(1): 6408.
- Al-Hussaniy HA, Alburghaif AH, Naji MA. Leptin hormone and its effectiveness in reproduction, metabolism, immunity, diabetes, hopes and ambitions. Journal of Medicine and Life 2021; 14(5): 600-605.
- Rovere C, Viale A, Nahon J, et al. Impaired processing of brain proneurotensin and promelanin-concentrating hormone in obese fat/fat mice. Endocrinology 1996; 137(7): 2954–2958.
- Lima FF, Sita LV, Oliveira AR, et al. Hypothalamic melanin-concentrating hormone projections to the septo-hippocampal complex in the rat. Journal of Chemical Neuroanatomy 2013; 47: 1-14.
- Rao Y, Lu M, Ge F, et al. Regulation of synaptic efficacy in hypocretin/orexin-containing neurons by melanin concentrating hormone in the lateral hypothalamus. The Journal of Neuroscience 2008; 28(37): 9101-9110.
- Vidal-Cevallos P, Mijangos-Trejo A, Uribe M, Tapia NC. The Interlink Between Metabolic-Associated Fatty Liver Disease and Polycystic Ovary Syndrome. Endocrinology and Metabolism Clinics of North America 2023; 52(3): 533-545.
- Altinkilic EM, du Toit T, Sakin Ö, et al. The serum steroid signature of PCOS hints at the involvement of novel pathways for excess androgen biosynthesis. The Journal of Steroid Biochemistry and Molecular Biology 2023; 233: 106366.
- Rajan L, Palaniswamy D, Mohankumar SK. Targeting obesity with plant-derived pancreatic lipase inhibitors: A comprehensive review. Pharmacological Research 2020; 155: 104681.
- Gunay YE, Kişioğlu SV, Karakullukçu S, et al. Comparison of orlistat and orlistat plus metformin therapy between diabetic and nondiabetic groups. Revista da Associacao Medica Brasileira (1992) 2023; 69(7): e20230174.
- LiverTox: Clinical and Research Information on Drug-Induced Liver Injury; National Institute of Diabetes and Digestive and Kidney Diseases: Bethesda, MD, USA, 2012.
- Khalil H, Ellwood L, Lord H, et al. Pharmacological Treatment for Obesity in Adults: An Umbrella Review. The Annals of Pharmacotherapy 2020; 54(7): 691-705.
- Jin X, Zhang ZH, Sun E, et al. Discussion on correlation between preparation, in vivo conversion process and potential structure-activity relationship of ginsenoside. China Zhongguo Zhongyao Zazhi 2013; 38(3): 307-313.
- Dai G, Wang J, Zheng J, et al. Bioactive polysaccharides from lotus as potent food supplements: a review of their preparation, structures, biological features and application prospects. Frontiers in Nutrition 2023; 10: 1171004.
- Zheng H, Han L, Shi W, et al. Research Advances in Lotus Leaf as Chinese Dietary Herbal Medicine. The American Journal of Chinese Medicine 2022; 50(6): 1423–1445.
- He Y, Tao Y, Qiu L, et al. Lotus (Nelumbo nucifera Gaertn.) Leaf-Fermentation Supernatant Inhibits Adipogenesis in 3T3-L1 Preadipocytes and Suppresses Obesity in High-Fat Diet-Induced Obese Rats. Nutrients 2022; 14(20).
- Kim BM, Cho BO, Jang SI. Anti-obesity effects of Diospyros lotus leaf extract in mice with high-fat diet-induced obesity. International Journal of Molecular Medicine 2019; 43(1): 603–613.
- Zhang Y, Ma L, Zhang L, et al. Effects and action mechanisms of lotus leaf (Nelumbo nucifera) ethanol extract on gut microbes and obesity in high-fat diet-fed rats. Frontiers in Nutrition 2023; 10.
- Ji L, Song T, Ge C, et al. Identification of bioactive compounds and potential mechanisms of scutellariae radix-coptidis rhizoma in the treatment of atherosclerosis by integrating network pharmacology and experimental validation. Biomedicine & Pharmacotherapy 2023; 165: 115210.
- Wang Y, Tao X, Gao Y, et al. Study on the mechanism of Shujin Tongluo granules in treating cervical spondylosis based on network pharmacology and molecular docking. Medicine 2023; 102(29): e34030.
- Hu X, Mola Y, Su WL, et al. A network pharmacology approach to decipher the total flavonoid extract of Dracocephalum Moldavica L. in the treatment of cerebral ischemia- reperfusion injury. PloS One 2023; 18(7): e0289118.
- Xu Z, Wang C, Luan Z, et al. Exploring the potential targets of the Abrus cantoniensis Hance in the treatment of hepatitis E based on network pharmacology. Frontiers in Veterinary Science 2023; 10: 1155677.
- Rajtar-Salwa R, Bobrowska B, Batko J, et al. Lipid-Lowering Therapy after Acute Coronary Syndrome in Outpatient Practice-How to Achieve Goal. Journal of Clinical Medicine 2023; 12(20).
- Yamaji T, Harada T, Kajikawa M, et al. Role of Small Dense Low-density Lipoprotein Cholesterol in Cardiovascular Events in Patients with Coronary Artery Disease and Type 2 Diabetes Mellitus Receiving Statin Treatment. Journal of Atherosclerosis and Thrombosis 2024; 31(4): 478-500.
- Farias-Pereira R, Savarese J, Yue Y, et al. Fat-lowering effects of isorhamnetin are via NHR-49-dependent pathway in Caenorhabditis elegans. Current Research in Food Science 2020; 2: 70-76.
- Liu L, Lan X, Chen X, et al. Multi-functional plant flavonoids regulate pathological microenvironments for vascular stent surface engineering. Acta Biomaterialia 2023; 157: 655–669.
- Deng HF, Wang XL, Sun H, et al. Puerarin inhibits expression of tissue factor induced by oxidative low-density lipoprotein through activating the PI3K/Akt/eNOS pathway and inhibiting activation of ERK1/2 and NF-κB. Life Sciences 2017; 191: 115–121.
- Li N, Wu X, Zhuang W, et al. Soy and Isoflavone Consumption and Multiple Health Outcomes: Umbrella Review of Systematic Reviews and Meta-Analyses of Observational Studies and Randomized Trials in Humans. Molecular Nutrition & Food Research 2020; 64(4).
- Ibarra M, Moreno L, Vera R, et al. Effects of the flavonoid quercetin and its methylated metabolite isorhamnetin in isolated arteries from spontaneously hypertensive rats. Planta Medica 2003; 69(11): 995-1000.
- Tsai SW, Lin CC, Lin SC, et al. Isorhamnetin ameliorates inflammatory responses and articular cartilage damage in the rats of monosodium iodoacetate-induced osteoarthritis. Immunopharmacology and Immunotoxicology 2019; 41(4): 504-512.
- González-Arceo M, Gomez-Lopez I, Carr-Ugarte H, et al. Anti-Obesity Effects of Isorhamnetin and Isorhamnetin Conjugates. International Journal of Molecular Sciences 2022; 24(1).
- Alqudah A, Qnais EY, Wedyan MA, et al. Isorhamnetin Reduces Glucose Level, Inflammation, and Oxidative Stress in High-Fat Diet/Streptozotocin Diabetic Mice Model. Molecules 2023; 28(2): 502.
- Jiang H, Yamashita Y, Nakamura A, et al. Quercetin and its metabolite isorhamnetin promote glucose uptake through different signalling pathways in myotubes. Scientific Reports 2019; 9(1): 2690.
- Nie N, Li Z, Li W, et al. Myricetin ameliorates experimental autoimmune myocarditis in mice by modulating immune response and inhibiting MCP-1 expression. European Journal of Pharmacology 2023; 942: 175549.
- Song X, Tan L, Wang M, et al. Myricetin: A review of the most recent research. Biomedicine & Pharmacotherapy 2021; 134: 111017.
- Liu K, Zhang Y, Zhang W, et al. A Study on the Interactions of Proteinase K with Myricetin and Myricitrin by Multi-Spectroscopy and Molecular Modeling. International Journal of Molecular Sciences 2023; 24(6): 5317.
- Meng Z, Wang M, Xing J, et al. Myricetin ameliorates atherosclerosis in the low-density-lipoprotein receptor knockout mice by suppression of cholesterol accumulation in macrophage foam cells. Nutrition & Metabolism 2019; 16: 25.
- Calzada F, Valdes M, Martínez-Solís J, et al. Annona cherimola Miller and Its Flavonoids, an Important Source of Products for the Treatment of Diabetes Mellitus: In Vivo and In Silico Evaluations. Pharmaceuticals 2023; 16(5): 724.
- Su H, Feng L, Zheng X, et al. Myricetin protects against diet-induced obesity and ameliorates oxidative stress in C57BL/6 mice. Journal of Zhejiang University Science B 2016; 17(6): 437-446.
- Liu IM, Liou SS, Lan TW, et al. Myricetin as the active principle of Abelmoschus moschatus to lower plasma glucose in streptozotocin-induced diabetic rats. Planta Medica 2005; 71(7): 617-621.
- Chen G, Xu H, Wu Y, et al. Myricetin suppresses the proliferation and migration of vascular smooth muscle cells and inhibits neointimal hyperplasia via suppressing TGFBR1 signaling pathways. Phytomedicine 2021; 92: 153719.
- Akindehin S, Jung YS, Kim SN, et al. Myricetin Exerts Anti-Obesity Effects through Upregulation of SIRT3 in Adipose Tissue. Nutrients 2018; 10(12): 1962.
- Barrios-Nolasco A, Domínguez-López A, Miliar-García A, et al. Anti-Inflammatory Effect of Ethanolic Extract from Tabebuia rosea (Bertol.) DC., Quercetin, and Anti-Obesity Drugs in Adipose Tissue in Wistar Rats with Diet-Induced Obesity. Molecules 2023; 28(9): 3801.
- Adeoluwa OA, Olayinka JN, Adeoluwa GO, et al. Quercetin abrogates lipopolysaccharide-induced depressive-like symptoms by inhibiting neuroinflammation via microglial NLRP3/NFκB/iNOS signaling pathway. Behavioural Brain Research 2023; 450: 114503.
- Ehteshami S, Abdollahi F, Ramezanian A, et al. Maintenance of quality and bioactive compounds of cold stored pomegranate (Punica granatum L.) fruit by organic acids treatment. Food Science and Technology International 2021; 27(2): 151-163.
- Della Via FI, Alvarez MC, Basting RT, et al. The Effects of Green Tea Catechins in Hematological Malignancies. Pharmaceuticals 2023; 16(7): 1021.
- Sarimahmut M, Celikler S. Plants from Northwestern Anatolia Display Selective Cytotoxicity and Induce Mitotic Catastrophe: A Study on Anticancer and Genotoxic Activities. Chemistry & Biodiversity 2023; 20(9): e202300460.
- Gerrard SD, Yonke JA, Seymour KA, et al. Feeding medium-chain fatty acid-rich formula causes liver steatosis and alters hepatic metabolism in neonatal pigs. American Journal of Physiology Gastrointestinal and Liver Physiology 2023; 325(2): G135-G146.
- Jiang LP, Sun HZ. Long-chain saturated fatty acids and its interaction with insulin resistance and the risk of nonalcoholic fatty liver disease in type 2 diabetes in Chinese. Frontiers in Endocrinology 2022; 13: 1051807.
- Takato T, Iwata K, Murakami C, et al. Chronic administration of myristic acid improves hyperglycaemia in the Nagoya-Shibata-Yasuda mouse model of congenital type 2 diabetes. Diabetologia 2017; 60(10): 2076-2083.
- Saraswathi V, Kumar N, Ai W, et al. Myristic Acid Supplementation Aggravates High Fat Diet-Induced Adipose Inflammation and Systemic Insulin Resistance in Mice. Biomolecules 2022; 12(6): 739.
- Ma R, Quan L, Aleteng QQ, et al. The impact of sitagliptin in palmitic acid-induced insulin resistance in human HepG2 cells through the suppressor of cytokine signaling 3/phosphoinositide 3-kinase/protein kinase B pathway. Journal of Physiology and Pharmacology 2023; 74(2).
- Yasuo T, Suwabe T, Sako N. Behavioral and Neural Responses to Vitamin C Solution in Vitamin C-deficient Osteogenic Disorder Shionogi/Shi Jcl-od/od Rats. Chemical Senses 2019; 44(6): 389-397.
- Wang X, Xu B, Du J, et al. Characterization of pyruvate metabolism and citric acid cycle patterns predicts response to immunotherapeutic and ferroptosis in gastric cancer. Cancer Cell International 2022; 22(1): 317.
- Muroyama K, Murosaki S, Yamamoto Y, et al. Anti-obesity effects of a mixture of thiamin, arginine, caffeine, and citric acid in non-insulin dependent diabetic KK mice. Journal of Nutritional Science and Vitaminology 2003; 49(1): 56-63.
- Jochym K, Kapusniak J, Barczynska R, et al. New starch preparations resistant to enzymatic digestion. Journal of the Science of Food and Agriculture 2012; 92(4): 886-891.
- Al-Samydai A, Al Qaraleh M, Al Azzam KM, et al. Formulating co-loaded nanoliposomes with gallic acid and quercetin for enhanced cancer therapy. Heliyon 2023; 9(6): e17267.
- Jafaripour L, Sohrabi Zadeh B, Jafaripour E, et al. Gallic acid improves liver cirrhosis by reducing oxidative stress and fibrogenesis in the liver of rats induced by bile duct ligation. Scandinavian Journal of Gastroenterology 2023; 58(12): 1474-1483.
- Bak EJ, Kim J, Jang S, et al. Gallic acid improves glucose tolerance and triglyceride concentration in diet-induced obesity mice. Scandinavian Journal of Clinical and Laboratory Investigation 2013; 73(8): 607-614.
- Duh PD, Lin SL, Wu SC. Hepatoprotection of Graptopetalum paraguayense E. Walther on CCl₄-induced liver damage and inflammation. Journal of Ethnopharmacology 2011; 134(2): 379-385.
- Wan Y, Xia J, Xu J, et al. Nuciferine, an active ingredient derived from lotus leaf, lights up the way for the potential treatment of obesity and obesity-related diseases. Pharmacological Research 2022; 175: 106002.
- Kim SM, Park EJ, Lee HJ. Nuciferine attenuates lipopolysaccharide-stimulated inflammatory responses by inhibiting p38 MAPK/ATF2 signaling pathways. Inflammopharmacology 2022;30(6): 2373-2383.
- Zhang Y, Li L, Zhang J, et al. Screening of hypolipidemic active components in Jiang-Zhi-Ning and its preliminary mechanism research based on "active contribution value" study. Journal of Ethnopharmacology 2021; 272: 113926.
- Liu CP, Kuo YC, Shen CC, et al. (S)-armepavine inhibits human peripheral blood mononuclear cell activation by regulating Itk and PLCgamma activation in a PI-3K-dependent manner. Journal of Leukocyte Biology 2007; 81(5): 1276-1286.
- Chen J, Ma X, Gao K, et al. The active ingredients of Jiang-Zhi-Ning: study of the Nelumbo nucifera alkaloids and their main bioactive metabolites. Molecules 2012; 17(8): 9855-9867.
- Wang YP, Zhang WB, Li HY, et al. Analysis on the route of conception vessel and governor vessel in Huangdi Neijing (The Yellow Emperor's Inner Classic). Zhongguo Zhen Jiu 2021; 41(7): 805-812.
- Wang J, Gu M. The skin diagnosis methods constructed by Liao Ping. Zhonghua Yi Shi Za Zhi 2023; 53(1): 28-35.
- Zhang GD, Chen Q, Tao TM, et al. Comparison of syndrome differentiation and treatment system between Huangdi Neijing and Treatise on Cold Damage. Asian Journal of Surgery 2023; 46(10): 4966-4700.
- Wei XT, Liu T, He ZJ, et al. Research progress in role of autophagy in diabetic wound healing and traditional Chinese medicine intervention. Zhongguo Zhong Yao Za Zhi 2023; 48(7): 1724-1730.
- Hoffman RD, Li CY, He K, et al. Chinese Herbal Medicine and Its Regulatory Effects on Tumor Related T Cells. Frontiers in Pharmacology 2020; 11: 492.
- Li Y, Li X, Li X, et al. Non-neglectable therapeutic options for age-related macular degeneration: A promising perspective from traditional Chinese medicine. Journal of Ethnopharmacology 2022; 282: 114531.