Main Text
1 Introduction
Andrographis paniculata [1-5], a plant steeped in ancient Asian traditional medicine, has earned its moniker as the "king of bitterness" in English, "Chiretteverte" and "Roi des amers" in French, "Kariyatu" in Gujarati, and "Kirayat" or "Kalpanath" in Hindi, with the colloquial name "kalmegh" in India [6,7]. This member of the Acanthaceae family thrives in various Asian regions, spanning tropical, subtropical, and southeastern locales, including India [8]. A. paniculata (AP) is predominantly used in the form of extracts and exhibits various pharmacological activities such as anti-bacterial [9], anti-viral [10], and immunosuppressant [11]. The main constituent of AP is andrographolide, which displays a wide range of biological activities including hepatoprotective, cardioprotective, antibacterial, antidiabetic, anti-inflammatory, antimalarial, and antitumor properties. In addition to these pharmacological benefits, AP has potential applications in treating rheumatoid arthritis. Over the last few decades, numerous andrographolide derivatives have emerged, and their pharmacological properties have been extensively studied [12]. Formulations based on nanotechnology also play a crucial role in biotechnology and phytochemical-based delivery. Burgos et al. conducted a study on the effectiveness of AP in relieving rheumatoid arthritis (RA) symptoms. They concluded that an AP formulation containing 30% andrographolide was effective against RA in clinical settings, although further studies are necessary [13]. Sandborn et al. examined the impact of AP extract on active ulcerative colitis. Their observations revealed that individuals with mild to moderately active ulcerative colitis who received AP extract (HMPL-004) at a daily dosage of 1,800 mg were more likely to achieve a clinical response compared to those who received a placebo [14]. Chiu et al. conducted a phase II clinical trial to assess the efficacy of AP water extract in palliative care for metastatic esophageal squamous cell cancer. They concluded that patients who completed AP therapy significantly lived longer survival periods and maintained their quality of life throughout the survival period compared to those who couldn't complete AP treatment [15]. We aimed to provide comprehensive information on the pharmacological activity of A. paniculata and its primary compound, andrographolide, in this work.
2 Chemistry of Andrographolide
Andrographolide is a bioactive compound found in the medicinal plant Andrographis paniculata is a diterpenoid lactone with a molecular formula C20H30O5 and a molar mass of approximately 350.45 g/mol. The IUPAC name of andrographolide is 3-[2-[decahydro-6-hydroxy-5-(hydroxymethyl)-5,8adimethyl-2-methylene-1-napthalenyl]ethylidene]dihydro-4-hydroxy-2(3H)-furanone [16].
It is a colorless, crystal-like, extremely bitter compound in taste. The bitterness is due to the presence of the lactone ring refs. The lactone ring is also responsible for the compound's biological activity refs. Andrographolide has been shown to inhibit the activity of several enzymes, including cyclooxygenase-2 (COX-2), which is involved in inflammation. Andrographolide has also been shown to inhibit the growth of cancer cells [17]. The chemical structure of andrographolide consists of a labdane diterpene backbone, containing a carbocyclic ring, an α, β-unsaturated-γ-lactone, two olefin bonds Δ8 and Δ12, and three hydroxyls at C-3, C-14, and C-19 (Figure 1). It is sparingly soluble in water but more soluble in organic solvents such as ethanol, methanol, chloroform, and ethyl acetate [18]. Andrographolide is relatively stable under normal conditions but can degrade when exposed to high temperatures, light, and moisture [19].
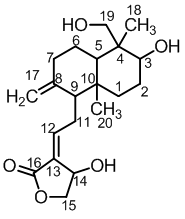
Figure 1 Structure of andrographolide.
The biosynthesis of andrographolide is a complex process that involves multiple pathways. The deoxy xylulose pathway (DXP) and mevalonate (MVA) pathway are the two main pathways involved in the production of this compound [20]. The DXP pathway begins with the condensation of two molecules of pyruvate to form methyl-D-erythritol-4-phosphate (MEP). MEP is then converted to several other compounds, including isopentenyl diphosphate (IPP) and dimethylallyl diphosphate (DMAPP). IPP and DMAPP are enzymatically condensed to form geranyl pyrophosphate (GPP), a C-10 isoprenoid compound refs. Then another condensation reaction involving GPP and IPP produces geranylgeranyl pyrophosphate (GGPP), a C20 isoprenoid compound. GGPP is then converted into various diterpene precursors through cyclization and rearrangement reactions. These precursors serve as the building blocks for the formation of different diterpenoid compounds in the plant refs. One of the diterpene precursors undergoes a cyclization reaction to form a labdane-type diterpene, specifically labdadienyl/copalyl diphosphate. This reaction is catalyzed by the enzyme ent-copalyl diphosphate synthase (CPS). The labdadienyl/copalyl diphosphate is further converted into andrographolide through a series of enzymatic reactions involving oxidation, rearrangement, and lactonization. Additionally, the MVA pathway begins with the condensation of three molecules of acetyl-CoA to form mevalonate. Mevalonate is then converted to IPP and DMAPP, which can then be used to synthesize a variety of diterpenes, including andrographolide refs.
The isolation of andrographolide from A. paniculata involves a series of steps [21]. Initially, the plant material is harvested and either dried or used fresh. The dried plant material, mainly leaf, is Grinded into a fine powder. The extraction of the leaf powder by cold maceration in a 1:1 mixture of dichloromethane and methanol is carried out and then andrographolide is directly isolated from the resulting extract by recrystallisation method [22]. Other solvents like ethanol, ethyl acetate, etc. can also be used for extraction purpose. Sometimes, the crude extract can also be purified using chromatography techniques. Finally, the presence and purity of andrographolide are confirmed through analytical techniques such as TLC, HPLC, NMR spectroscopy, and mass spectrometry [23].
Although, andrographolide has been recognized for its potential therapeutic properties, one of the significant challenges associated with its use as a therapeutic agent is its poor bioavailability. Suresh et al. have tried to solve this problem by developing a cocrystal for andrographolide [24]. The study revealed that andrographolide-salicylic acid cocrystal proved to be particularly promising as it completely inhibited the chemical transformation of andrographolide to its inactive sulfate metabolite. Additionally, the cocrystal showed a significantly faster dissolution rate and higher drug release compared to pure andrographolide. This discovery may offer a potential solution to enhance the efficacy and pharmaceutical properties of andrographolide as a herbal medicine [24].
3 Applications of Andrographolide
The Acanthaceae family of plants includes A. paniculata [25]. This herb has been used for many years to treat several diseases conditions such as colds, laryngitis malaria, fever, diarrhea, hypertension, and diabetes [26]. Various pharmacological properties of this herb have been already documented such as antiviral, antibacterial, hypocholesterolemic, hypoglycaemic, and adaptogenic [27]. The diterpene lactone andrographolide was the most prominent biologically active chemical of the plant. It has been reported that andrographolide has a variety of pharmacological effects such as immunostimulatory, anti-inflammatory, antidiarrheal, anti-cancer, anti-microbial, antihepatitic, cardioprotective, antihyperglycemic anti-microbial, antioxidant , and cardiovascular [28]. Currently, techniques for extracting and assessing andrographolide from a diverse range of herbal mixtures, complex compositions, and biological sources have been developed. These techniques include Electroanalytical methods, Chemiluminescence methods, Spectrophotometry, and Chromatography. These methodologies are utilized for the quality analysis of biological samples and pharmaceutical formulations [29].
3.1 Immunity enhancer
In various traditional medicine practices across the globe, A. paniculata, a plant of medicinal value, has been recognized and utilized as a powerful herbal medicine to treat a variety of health conditions [30]. Andrographolide, an active constituent of the plant has been shown to improve non-specific immunity by promoting lymphocyte phagocytosis and replication as well as specific immunity, such as antibody reactions and delayed-type hypersensitivity response [31]. The formulation of Andrographolide sulfonate has been reported to directly modulate the immune response of T-lymphocytes and neutrophils, thereby enhancing the efficiency of the host's immune response in treating various disease conditions [32]. Andrographolide is an immunostimulant agent that may influence immune function by triggering macrophages, natural killer cells, and cytokines [33]. By reducing T-cell and antibody responses to myelin antigens, andrographolide treatment markedly reduced experimental autoimmune encephalomyelitis symptoms in mice (Figure 2). In vivo experimental results showed that AGL can inhibit T-cell activation in vitro and may help to reduce negative T-cell reactions [34]. Andrographolide alters the mitogen-activated protein kinase (MAPK)-Nrf2-HO-1 signaling cascade in cerebral endothelial cells and provides a protective effect in ischemic stroke in rats [35].
3.2 Hepatoprotective
The primary active anti-hepatotoxic compound in A. paniculata is andrographolide [36]. Numerous biological activities of andrographolide have been related to reports of its therapeutic and preventative benefits on liver disorders. It effectively prevents liver injury induced by external factors, which may be due to oxidative stress and inflammatory reactions [37]. Tang et al. have synthesized derivatives of andrographolide with increased water solubility and assessed for hepatoprotective potency. The outcomes of the experiment suggested that adequate aqueous solubility may enhance the drug absorption and bioavailability, which indirectly increases their hepatoprotective effect in mice [38]. Chen et al. have reported potential in vitro and in vivo activity of synthetic derivatives of andrographolide for the treatment of liver damage. These derivatives of andrographolide effectively prevent chemically induced acute liver injury by blocking STAT3 (Signal Transducer and Activator of Transcription 3) activity [39]. Additionally, it has been observed that nano-formulated andrographolide particles exhibit hepatoprotective activities [40]. Polylactide co-glycolide nano capsulated andrographolide was well reported with enhanced hepatoprotective potency compared to free andrographolide against liver damage inflicted by the poison arsenic [41].
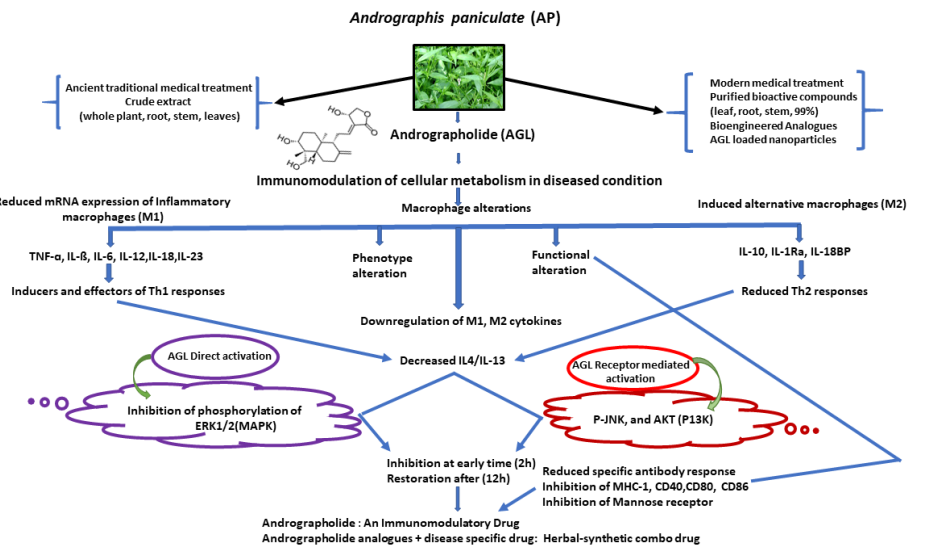
Figure 2 Diagram depicting the effects of andrographolide and a potential mechanism for regulation of the immune system. (This figure adopted CCBY4 from reference [42]).
3.3 Cardioprotective
Andrographolide reduces LPS-induced dysfunction of the heart in mice by inhibiting TNF-α, IL-1β, IκB phosphorylation, and NO (nitric oxide) production, myocardial apoptosis and hence offering a potential treatment for myocardial dysfunction caused by sepsis [43]. Zhang et al. investigated the impact of andrographolide on Lewis’s rat models of experimental autoimmune myocarditis. Their study revealed that andrographolide treatment led to reduced levels of TNF-α, IL-17, and myosin antibodies. Moreover, it inhibited the infiltration of CD3+ positive T cells and CD14+ positive monocytes in myocarditis rats. The protective effects of andrographolide proposed suppression of cardiac inflammation and blockade of the PI3K/Akt pathway [44]. Andrographolide was also reported to improve cardiac function in diabetic mice. The experimental result reported with andrographolide reduces diabetes-induced cardiac hypertrophy and blocks NF-κB activation, protein expression of IL-6, and adhesion molecules. As suppressing NF-κB-mediated inflammation and modulated NOXs/Nrf2-mediated oxidative stress andrographolide was found promising compound for the treatment of diabetic cardiomyopathy [45]. A 50 mg/kg dose of andrographolide protects against high-fat diet-induced cardiac damage in mice by inhibiting apoptosis and by efficiently improving the IGF-1R (Insulin like Grwth Factor–1R) compensatory mechanism and significantly supporting the efficacy of andrographolide supplementation in managing the symptoms of cardiovascular disease in obese people [46].
In neonatal rat cardiomyocytes, Woo et al. observed the cardioprotective action of andrographolide against reoxygenation/hypoxic damage, upregulating antioxidant enzyme activities and lowering cellular glutathione levels [47]. By boosting NF-E2–related factor 2 expression both in vivo and in vitro, andrographolide plays a crucial role in heart protection by defending against oxidative stress upon myocardial infarction and subsequently enhanced cardiac performance [48]. The study by Shu et al. explored the favourable impact of andrographolide on a mouse model of coronary heart disease. This effect was achieved by inhibiting the expression of Peroxisome Proliferator-Activated Receptors (PPARs) and the NF-κB signaling pathways [49]. Beneficial effect of andrographolide was also reported on adverse cardiac remodelling after myocardial infarction through reducing oxidative stress by enhancing the expression of Nrf2 [48].
3.4 Anti-inflammatory properties of Andrographolide
Andrographolide's anti-inflammatory effects were attributed to its multifaceted mechanisms of action. A key player in inflammation regulation is the NF-κB signaling pathway, which andrographolide prominently inhibits by blocking its activation and translocation into the nucleus [50]. NF-κB regulates the expression of various pro-inflammatory genes, including cytokines like interleukin-6 (IL-6) and tumor necrosis factor-alpha (TNF-α), as well as enzymes like cyclooxygenase-2 (COX-2) [51]. By impeding NF-κB, andrographolide leads to reduced expression of pro-inflammatory cytokines, contributing to the attenuation of the inflammatory response at the molecular level.
Andrographolide's interference with the NF-κB pathway extends to COX-2, a crucial enzyme involved in prostaglandin synthesis. Prostaglandins are lipid mediators that contribute to inflammation, pain, and fever [52]. Andrographolide's ability to suppress COX-2 expression leads to diminished production of prostaglandins, further contributing to the attenuation of the inflammatory response at the molecular level (Figure 3). These coordinated actions of andrographolide result in the effective regulation of inflammation, modulating the inflammatory environment and preventing the overactivation of immune responses that can lead to tissue damage and chronic inflammation [53].
Moreover, andrographolide exerts its anti-inflammatory prowess by modulating the activity of immune cells. Macrophages play a central role in the initiation and resolution of inflammation [54]. Andrographolide promotes the polarization of macrophages toward an anti-inflammatory M2 phenotype, reducing their pro-inflammatory cytokine secretion while enhancing the secretion of anti-inflammatory mediators [55]. Similarly, Andrographolide affects T-cells, promoting the differentiation of regulatory T-cells (Tregs), which have anti-inflammatory properties and help regulate immune responses [53]. By modulating these immune cells, Andrographolide further contributes to its anti-inflammatory effects.
Andrographolide's potent antioxidant activity also plays a major role in its anti-inflammatory actions [56]. Inflammation often leads to the generation of reactive oxygen species (ROS) and oxidative stress, which can further exacerbate tissue damage and inflammation [57]. Andrographolide's potent antioxidant effects help neutralize these harmful ROS, thereby mitigating oxidative stress and its detrimental effects on cells and tissues.
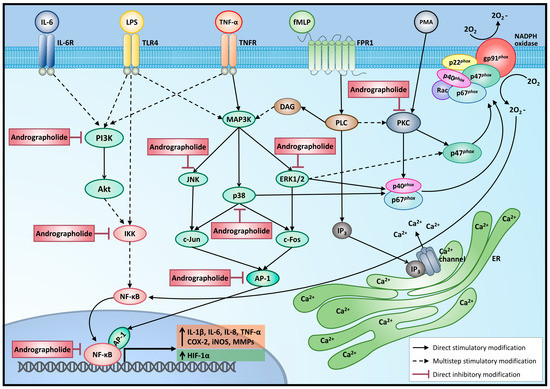
Figure 3 Anti-inflammatory effects of Andrographolide (Adopted under CC BY from [58]).
3.5 Anti-cancer property of Andrographolide
Andrographolide's anti-cancer actions encompass various stages of cancer development, from halting uncontrolled cell proliferation to interfering with the formation of new blood vessels crucial for tumor growth and metastasis [59].
One of the central aspects of its anti-cancer activity lies in its ability to impede cancer cell proliferation. Andrographolide achieves this by targeting the cell cycle and disrupting the intricate regulatory checkpoints that control cell division. Through the downregulation of key cell cycle regulatory proteins such as cyclins and cyclin-dependent kinases (CDKs), Andrographolide induces cell cycle arrest at specific phases, such as the G0/G1, S, or G2/M phases [60]. This arrest prevents cancer cells from continuously dividing and accumulating, effectively inhibiting tumor growth. By interfering with the cell cycle progression, andrographolide effectively thwarts the uncontrolled proliferation characteristic of cancer cells, offering a potential means of curbing tumor progression [61].
Moreover, andrographolide's induction of apoptosis (programmed cell death) represents a pivotal mechanism in its anti-cancer effects [60,62]. Cancer cells often evade apoptosis, allowing them to survive and multiply uncontrollably. However, andrographolide addresses this evasion by triggering a series of cellular events that promote apoptosis. It upregulates pro-apoptotic proteins like Bax and activates caspases, the enzymes responsible for carrying out the process of apoptosis. Concurrently, it downregulates anti-apoptotic proteins like Bcl-2, which counteract the pro-apoptotic signals. These coordinated actions tip the balance in favor of apoptosis, prompting cancer cells to undergo programmed self-destruction. This pro-apoptotic activity of Andrographolide contributes significantly to its anti-cancer efficacy and provides a means to selectively eliminate cancer cells [62,63].
Angiogenesis, the process by which new blood vessels sprout from existing ones, is essential for tumor survival and growth. Tumors exploit this process to create a network of blood vessels that supply them with nutrients and oxygen [64]. However, andrographolide disrupts this advantageous network by suppressing the expression of vascular endothelial growth factor (VEGF) and matrix metalloproteinases (MMPs). VEGF is a potent pro-angiogenic factor that stimulates blood vessel formation, while MMPs play a role in breaking down the extracellular matrix, allowing new blood vessels to grow. By inhibiting VEGF and MMPs, andrographolide impedes the growth of new blood vessels, thereby depriving tumors of their essential lifeline for sustenance [65]. As a consequence, the tumor's ability to grow and metastasize is compromised.
Overall, andrographolide's capacity to modulate critical pathways involved in cancer progression makes it an attractive candidate for further exploration in cancer research and drug development. While preclinical studies and in vitro experiments have provided compelling evidence of its anti-cancer efficacy, additional investigations, including clinical trials, are essential to fully unlock its therapeutic potential and establish its safety and efficacy as a part of cancer treatment strategies.
3.6 Upper respiratory tract infection
Upper respiratory tract infections (URTIs) are widespread globally, leading to substantial morbidity and imposing a substantial economic burden. The escalating concern over antibiotic resistance has spurred interest in alternative therapies [66]. Andrographolide has emerged as a promising remedy for managing URTIs.
Andrographolide expertly influences the immune response by enhancing the body's defense mechanisms against both viral and bacterial infections. It stimulates the activity of vital immune cells, such as macrophages and natural killer (NK) cells, which serve as crucial sentinels in identifying and eliminating invading pathogens [55,67].
Extensive studies have revealed that Andrographolide possesses potent antiviral activity, encompassing a wide spectrum of viruses, including respiratory viruses like influenza viruses and coronaviruses. It exhibits its antiviral prowess by disrupting viral replication and interfering with viral entry into host cells. Consequently, the viral load is significantly reduced, resulting in a milder and less severe manifestation of URTI symptoms [68].
URTIs are frequently accompanied by an inflammatory response in the upper respiratory tract, culminating in distressing symptoms such as sore throat, nasal congestion, and cough [69]. Notably, Andrographolide's anti-inflammatory properties play a pivotal role in alleviating these discomforts. By downregulating the production of pro-inflammatory cytokines and mediators, Andrographolide mitigates the intensity of the inflammatory response. This dampening effect on inflammation significantly contributes to the relief of URTI-related symptoms [53].
Andrographolide exhibits several properties that make it a promising asset for the treatment of URTIs. This includes broad spectrum antiviral activity [70], Immune system modulation [67], Anti-inflammatory [53] and anti-bacterial activities [71].
4 Preclinical and clinical studies on Andrographolide
Several clinical and preclinical trials on andrographolide have been completed, revealing significant results across various conditions and diseases, including cardiovascular issues, rheumatoid arthritis, cancer, upper respiratory tract infections, inflammation, and more. Andrographolide (ADP) has proven effective in boosting the immune system at specific concentrations [12]. In the study conducted by Aldurrah et al. [72] the anti-depressant effects of andrographolide were evaluated using chronic unpredictable stress (CUS) zebrafish model. Zebrafishes were subjected to CUS, and then treated with CUS + Andrographolide (100 mg/L) and CUS + fluoxetine (0.01 mg/L). After inducing stress, zebrafishes’ behaviours and potential toxicity effects were assessed within a 24-hour period. The study's findings revealed an increase in the total distance travelled, and importantly, andrographolide exhibited a significant reduction in freezing duration. Indirapriyadarshinni et al. [73] studied an effect of andrographolide in UV-B induced mouse skin. A group of mice were subjected to UVB radiation at the dose of 180 mL/cm2 for 10 days. The drug andrographolide (3.6mg kg/b.Wt) was topically administered 1 hour before each UVB radiation. The pretreatment with the drug reduced lipid peroxidation and restored antioxidant status in mouse skin. Further, it slowed down the UVB-induced inflammatory cytokines such as CD34, iNOS, NF-Κb, COX-2, IL-6, 10 in the mice skin. Yang et al. [74] conducted a study demonstrating andrographolide supresses aerobic glycolysis and triggers cell death in cancer cells. It exhibited anticancer activity by inhibiting pyruvate dehydrogenase kinase 1 expression in lung cancer cells. This is achieved through cleavage of polymerase, activation of caspase 3, and damage to the mitochondria, resulting in increased reactive oxygen species. Further details of these clinical trials are provided in Table 1.
Table 1 Summary of clinical trials of Andrographolide.
| NCT number | Other names | Phase | Study design | Conditions | Volunteer | Outcome measures |
|---|---|---|---|---|---|---|
| NCT02273635 | 14PIE-26946CORFO14-391 | Phase 1 Phase 2 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment | Primary Progressive Multiple Sclerosis Multiple Sclerosis, Secondary Progressive | 68 |
|
| NCT04196075 | CRE-2017.616 | Phase 3 | Allocation: N/A Intervention Model: Single Group Assignment Masking: None (Open Label) Primary Purpose: Supportive Care | Squamous Cell Carcinoma of Esophagus | 30 |
|
| NCT03262792 | VL/170105/PA/OA | Not Applicable | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Investigator, Outcomes Assessor) Primary Purpose: Treatment | Knee Osteoarthritis | 108 |
|
| NCT03455049 | IndonesiaU-02 | Not Applicable | Allocation: Randomized Intervention Model: Crossover Assignment Masking: Double (Participant, Investigator) Primary Purpose: Treatment | Increased Insulin | 73 |
|
| NCT02280876 | PaCRU-02/PCNS. EM/12PCNS-EM | Phase 1 Phase 2 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment | Multiple sclerosis, relapsing remitting | 30 |
|
| NCT04463875 | Corfu HC | - | Observational Model: Case-Only Time Perspective: Prospective | Migraine | 113 |
|
| NCT03780621 | EP-1004 | Phase 1 | Allocation: Randomized Intervention Model: Crossover Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment | Cognitive Impairment, Mild | 16 |
|
| NCT00749645 | PCT06-AG-02DO4I1240FONDEF | Phase 2 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Quadruple (Participant, Care Provider, Investigator, Outcomes Assessor) Primary Purpose: Treatment | Arthritis, Rheumatoid (AR) | 60 |
|
| NCT04955327 | HP/200802/PARACTIN/CC | Phase 3 | Allocation: Randomized Intervention Model: Parallel Assignment Masking: Triple (Participant, Care Provider, Investigator) Primary Purpose: Treatment | Upper Respiratory Tract Infection | 225 |
|
5 Side effects and toxicity of Andrographolide
The administration of andrographolide is linked to a spectrum of common side effects, encompassing various categories. Gastrointestinal disturbances stand out as the most prevalent, including discomforts like nausea, vomiting, diarrhea, and abdominal pain [79]. Another notable side effect is the occurrence of headaches [80]. Some individuals administered with andrographolide have reported skin rashes [79]. Additional side effects include dysgeusia [79] and alterations in libido [81]. In more rare circumstances, andrographolide has been associated with more serious side effects, such as anaphylactic reactions, which represent severe and potentially life-threatening allergic responses [79]. Furthermore, the administration of andrographolide has been linked to the possibility of causing renal damage in select individuals [82]. The presence of these rare but significant side effects emphasizes the importance of close monitoring and immediate medical attention. The wide range of potential side effects underscores the need for careful evaluation and personalized risk assessment when considering andrographolide as part of a therapeutic regimen.
Earlier experiments of andrographolide using animal models were reported without any serious toxicity [83,84]. However recent reported studies have shown toxicity associated with andrographolide is not negligible. Huang et al. examined the effect of andrographolide on the embryonic stem cell test model and reported the reproductive toxicity increasing ROS level, potential damage to the mitochondrial membrane, and interfering caspase-3 and nuclear factor 2 related factor 2 protein [85]. Male reproductive toxicity of andrographolide was examined using a male albino rat model and the result showed a disruption of somniferous epithelium and abnormalities in sperm count and motility [86]. Andrographolide is well reported with nephrotoxicity and case studies also support the nephrotoxicity with a reduction in urine output and acute tubular necrosis in patients [87]. Observations reported as andrographolide activates endoplasmic reticulum stress signaling by increasing the expressions of C/EBP homologous protein and Caspase-4. This endoplasmic reticulum stress and TNF-α, and IL-6-induced inflammations can be a key mechanism for the nephrotoxic effect of Andrographolide.3 Further studies identified the potential toxicity biomarkers such as 2-ketoadipate and 1,5-anhydroglucitol which conform metabolic changes due to Andrographolide induced nephrotoxicity [88]. Safety profile of andrographolide extract demonstrated with no cytotoxicity with 13.2-81.5 μM range of andrographolide content on human cell lines sampled from sensitive organs including liver, brain, lungs, and intestine [89].
In a notable study conducted by Batran et al., it was observed that Andrographolide exhibits a remarkable level of safety, particularly in the context of toxicity, as evidenced by their research findings. In their comprehensive investigation, the researchers administered Andrographolide to rats at a significant dosage of 500 mg/kg/day. The outcomes of this study affirmatively indicate that Andrographolide is characterized by a lack of toxicity at this substantial dosage level [90].
However, it is imperative to note that certain populations warrant circumspect usage of Andrographolide. For instance, prudent caution is advised against its consumption by pregnant or breastfeeding women due to considerations of potential adverse effects [91]. Furthermore, a corpus of literature signifies that individuals grappling with hepatic or renal ailments ought to exercise restraint in its utilization. Similarly, individuals concurrently prescribed specific medications, such as anticoagulants or immunosuppressants, should approach its utilization judiciously [92]. This caveat is underscored by the intricate interplay between Andrographolide and specific medications, which necessitates a vigilant assessment of potential contraindications and interactions to ensure optimal therapeutic outcomes and safety considerations.
6 Conclusion and future prospects
Numerous researchers have been drawn to andrographolide due to its remarkable pharmacological properties. To enhance its biological activities, diverse derivatives of andrographolide have been synthesized. In this article, we aim to provide a comprehensive summary of various experimental and clinical trials exploring its applications, including as an antioxidant, immunity enhancer, hepatoprotective agent, cardioprotective agent, anti-inflammatory agent, anti-cancer compound, and treatment for upper respiratory tract infections. Given the increasing global prevalence of inflammation, there is a pressing need for the development of highly effective anti-inflammatory medications Andrographolide, a chemical constituent of Andrographis paniculata, holds promise in this regard. Research conducted across various systemic disorders has demonstrated andrographolide's anti-inflammatory effects. Its potential extends to respiratory, digestive, immune, cardiovascular, nervous, and skeletal system disorders, as well as tumors and other inflammatory conditions. While existing knowledge is promising, further studies are essential to delve into the details and gain a deeper understanding of the diverse applications of andrographolide.
Back Matter
Acknowledgments
Not applicable.
Conflicts of Interest
The authors declare no conflicts of interest.
Author Contributions
Conceptualization, Divya Teli and Dixa A. Vaghela; Data curation,Amit Chaudhri; Formal analysis, Hetvi K. Solanki; Methodology, Keshav Jetha; Writing-Original draft, Vivek P. Chavda and Divya Teli; Writing-review and editing, Dixa A. Vaghela and Amit Chaudhri; All authors have read and agreed to the published version of the manuscript.
Ethics Approval and Consent to Participate
The study was approved by the Medical Ethics Committee, and the patients were informed and consented.
Funding
This research received no external funding.
Availability of Data and Materials
The data presented in this study are available on request from the corresponding author.
Supplementary Materials
Not applicable.
References
- Chavda VP, Kumar A, Banerjee R, et al. Ayurvedic and Other Herbal Remedies For Dengue: An Update. Clinical Complementary Medicine and Pharmacology 2022; 2(3): 100024.
- Chavda VP, Patel AB, Mistry KJ, et al. Nano-Drug Delivery Systems Entrapping Natural Bioactive Compounds for Cancer: Recent Progress and Future Challenges. Frontiers in Oncology 2022; 12: 867655.
- Chavda VP, Gajjar N, Shah N, et al. Darunavir ethanolate: Repurposing an anti-HIV drug in COVID-19 treatment. European Journal of Medicinal Chemistry Reports 2021; 3: 100013.
- Chavda VP, Patel AB, Vihol D, et al. Herbal Remedies, Nutraceuticals, and Dietary Supplements for COVID-19 Management: An Update. Clinical Complementary Medicine and Pharmacology 2022; 2(1): 100021.
- Khadela A, Chavda VP, Postwala H, et al. Epigenetics in Tuberculosis: Immunomodulation of Host Immune Response. Vaccines 2022; 10(10): 1740.
- Akbar S. Andrographis paniculata: A review of pharmacological activities and clinical effects. Alternative Medicine Review 2011; 16(1): 66-77.
- Hossain MS, Urbi Z, Sule A, et al. Andrographis paniculata (Burm. f.) Wall. ex Nees: A Review of Ethnobotany, Phytochemistry, and Pharmacology. The Scientific World Journal 2014; 2014: 274905.
- Kumar RA, Sridevi K, Kumar NV, et al. Anticancer and immunostimulatory compounds from Andrographis paniculata. Journal of Ethnopharmacology 2004; 92(2–3): 291–295.
- Singha PK, Roy S, Dey S. Antimicrobial activity of Andrographis paniculata. Fitoterapia 2003; 74(7–8): 692–694.
- Calabrese C, Berman SH, Babish JG, et al. A phase I trial of andrographolide in HIV positive patients and normal volunteers. Phytotherapy Research 2000; 14(5): 333–338.
- Rajagopal S, Kumar RA, Deevi DS, et al. Andrographolide, a potential cancer therapeutic agent isolated from Andrographis paniculata. Journal of Experimental Therapeutics and Oncology 2003; 3(3): 147–158.
- Jayakumar T, Hsieh CY, Lee JJ, et al. Experimental and Clinical Pharmacology of Andrographis paniculata and Its Major Bioactive Phytoconstituent Andrographolide. Evidence-Based Complementary and Alternative Medicine 2013; 2013: 846740.
- Burgos RA, Hancke JL, Bertoglio JC, et al. Efficacy of an Andrographis paniculata composition for the relief of rheumatoid arthritis symptoms: a prospective randomized placebo-controlled trial. Clinical Rheumatology 2009; 28(8): 931–946.
- Sandborn WJ, Targan SR, Byers VS, et al. Andrographis paniculata Extract (HMPL-004) for Active Ulcerative Colitis. American Journal of Gastroenterology 2013; 108(1): 90–98.
- Chiu PWY, Yue GGL, Cheung MK, et al. The effect of Andrographis paniculata water extract on palliative management of metastatic esophageal squamous cell carcinoma—A phase II clinical trial. Phytotherapy Research 2023; 37(8): 3438-3452.
- Yan Y, Fang LH, Du GH. Andrographolide. In Natural Small Molecule Drugs from Plants; Springer Singapore: Gateway East, Singapore, 2018; pp. 357–362.
- Owen AE, Louis H, Ejiofor EU, et al. Natural Andrographolide Isolated from Andrographis paniculata as Potent Epileptic Agent: Spectroscopy, Molecular Structure, and Molecular Docking Investigation. Chemistry Africa 2023; 6: 2445-2461.
- Smith AB, Toder BH, Carroll PJ, et al. Andrographolide: an X-ray crystallographic analysis. Journal of Crystallographic and Spectroscopic Research 1982; 12(4): 309–319.
- Lomlim L, Jirayupong N, Plubrukarn A. Heat-accelerated degradation of solid-state andrographolide. Chemical & Pharmaceutical Bulletin 2003; 51(1): 24-26.
- Srivastava N, Akhila A. Biosynthesis of andrographolide in Andrographis paniculata. Phytochemistry 2010; 71(11–12): 1298–1304.
- Chao WW, Lin BF. Isolation and identification of bioactive compounds in Andrographis paniculata (Chuanxinlian). Chinese Medicine 2010; 5(1): 17.
- Rajani M, Shrivastava N, Ravishankara MN. A rapid method for isolation of andrographolide from andrographis paniculata nees (kalmegh). Pharmaceutical Biology 2000; 38(3): 204–209.
- Hao M, Lv M, Xu H. Andrographolide: Synthetic Methods and Biological Activities. Mini-Reviews in Medicinal Chemistry 2020; 20(16): 1633–1652.
- Suresh K, Goud NR, Nangia A. Andrographolide: Solving Chemical Instability and Poor Solubility by Means of Cocrystals. Chemistry – An Asian Journal 2013; 8(12): 3032–3041.
- Boopathi CA. Andrographis SPP.: A Source of Bitter Compounds for Medicinal Use. Ancient Science of Life 2000; 19(3–4): 164–168.
- Mishra SK, Sangwan NS, Sangwan RS. Phcog Rev.: Plant Review Andrographis paniculata (Kalmegh): A Review. Pharmacognosy Reviews 2007; 1(2): 283-298.
- Bhatnagar SS, Santapau H, Desa JD, et al. Biological activity of Indian medicinal plants. I. Antibacterial, antitubercular and antifungal action. Indian Journal of Medical Research 1961; 49: 799–813.
- Kumar G, Singh D, Tali JA, et al. Andrographolide: Chemical modification and its effect on biological activities. Bioorganic Chemistry 2019; 95: 103511.
- Patil R, Jain V. Andrographolide: A Review of Analytical Methods. Journal of Chromatographic Science 2021; 59(2): 191–203.
- Okhuarobo A, Ehizogie Falodun J, Erharuyi O, et al. Harnessing the medicinal properties of Andrographis paniculata for diseases and beyond: a review of its phytochemistry and pharmacology. Asian Pacific Journal of Tropical Disease 2014; 4(3): 213–222.
- Naik S, Hule A. Evaluation of Immunomodulatory Activity of an Extract of Andrographolides from Andographis paniculata. Planta Medica 2009; 75(08): 785–791.
- Li M, Yang X, Guan C, et al. Andrographolide sulfonate reduces mortality in Enterovirus 71 infected mice by modulating immunity. International Immunopharmacology 2017; 55: 142–150.
- Peng G, Zhou F, Ding R, et al. Modulation of lianbizi injection (andrographolide) on some immune functions. Zhongguo Zhong Yao Za Zhi 2002; 27(2): 147–150.
- Iruretagoyena MI, Tobar JA, González PA, et al. Andrographolide Interferes with T Cell Activation and Reduces Experimental Autoimmune Encephalomyelitis in the Mouse. Journal of Pharmacology and Experimental Therapeutics 2005; 312(1): 366–372.
- Yen TL, Chen RJ, Jayakumar T, et al. Andrographolide stimulates p38 mitogen-activated protein kinase–nuclear factor erythroid-2-related factor 2–heme oxygenase 1 signaling in primary cerebral endothelial cells for definite protection against ischemic stroke in rats. Translational Research 2016; 170: 57–72.
- Handa S, Sharma A. Hepatoprotective activity of andrographolide from Andrographis paniculata against carbontetrachloride. Indian Journal of Medical Research 1990; 92: 276–283.
- Ye JF, Zhu H, Zhou ZF, et al. Protective Mechanism of Andrographolide against Carbon Tetrachloride-Induced Acute Liver Injury in Mice. Biological & Pharmaceutical Bulletin 2011; 34(11): 1666–1670.
- Tang C, Gu G, Wang B, et al. Design, Synthesis, and Biological Evaluation of Andrographolide Derivatives as Potent Hepatoprotective Agents. Chemical Biology & Drug Design 2014; 83(3): 324–333.
- Chen SR, Li F, Ding MY, et al. Andrographolide derivative as STAT3 inhibitor that protects acute liver damage in mice. Bioorganic & Medicinal Chemistry 2018; 26(18): 5053–5061.
- Roy P, Das S, Auddy RG, et al. Engineered Andrographolide Nanoparticles Mitigate Paracetamol Hepatotoxicity in Mice. Pharmaceutical Research 2013; 30(5): 1252–1262.
- Das S, Pradhan GK, Das S, et al. Enhanced protective activity of nano formulated andrographolide against arsenic induced liver damage. Chemico-Biological Interactions 2015; 242: 281–289.
- Mishra A, Shaik HA, Sinha RK, et al. Andrographolide: A Herbal-Chemosynthetic Approach for Enhancing Immunity, Combating Viral Infections, and Its Implication on Human Health. Molecules 2021; 26(22): 7036.
- Zhang J, Zhu D, Wang Y, et al. Andrographolide Attenuates LPS-Induced Cardiac Malfunctions Through Inhibition of IκB Phosphorylation and Apoptosis in Mice. Cellular Physiology and Biochemistry 2015; 37(4): 1619–1628.
- Zhang Q, Hu L, Li H, et al. Beneficial effects of andrographolide in a rat model of autoimmune myocarditis and its effects on PI3K/Akt pathway. The Korean Journal of Physiology & Pharmacology 2019; 23(2): 103.
- Liang E, Liu X, Du Z, et al. Andrographolide Ameliorates Diabetic Cardiomyopathy in Mice by Blockage of Oxidative Damage and NF-κB-Mediated Inflammation. Oxidative Medicine and Cellular Longevity 2018; 2018: 9086747.
- Lin KH, Asokan SM, Kuo WW, et al. Andrographolide mitigates cardiac apoptosis to provide cardio‐protection in high‐fat‐diet‐induced obese mice. Environmental Toxicology 2020; 35(6): 707–713.
- Woo AYH, Waye MMY, Tsui SKW, et al. Andrographolide Up-Regulates Cellular-Reduced Glutathione Level and Protects Cardiomyocytes against Hypoxia/Reoxygenation Injury. Journal of Pharmacology and Experimental Therapeutics 2008; 325(1): 226–235.
- Xie S, Deng W, Chen J, et al. Andrographolide Protects Against Adverse Cardiac Remodeling After Myocardial Infarction through Enhancing Nrf2 Signaling Pathway. International Journal of Biological Sciences 2020; 16(1): 12–26.
- Shu J, Huang R, Tian Y, et al. Andrographolide protects against endothelial dysfunction and inflammatory response in rats with coronary heart disease by regulating PPAR and NF-κB signaling pathways. Annals of Palliative Medicine 2020; 9(4): 1965–1975.
- Mishra K. Andrographolide: Regulating the Master Regulator NF-κB. Indian Journal of Clinical Biochemistry 2021; 36(1): 117–119.
- Liu T, Zhang L, Joo D, et al. NF-κB signaling in inflammation. Signal Transduction and Targeted Therapy 2017; 2: 17023.
- Ricciotti E, FitzGerald GA. Prostaglandins and Inflammation. Arteriosclerosis, Thrombosis, and Vascular Biology 2011; 31(5): 986–1000.
- Li X, Yuan W, Wu J, et al. Andrographolide, a natural anti-inflammatory agent: An Update. Frontiers in Pharmacology 2022; 13: 920435.
- Fujiwara N, Kobayashi K. Macrophages in inflammation. Current Drug Targets - Inflammation & Allergy 2005; 4(3): 281–286.
- Wang W, Wang J, Dong S, et al. Immunomodulatory activity of andrographolide on macrophage activation and specific antibody response. Acta Pharmacologica Sinica 2010; 31(2): 191–201.
- Mussard E, Cesaro A, Lespessailles E, et al. Andrographolide, A Natural Antioxidant: An Update. Antioxidants 2019; 8(12): 571.
- Schieber M, Chandel NS. ROS Function in Redox Signaling and Oxidative Stress. Current Biology 2014; 24(10): R453–R462.
- Burgos RA, Alarcón P, Quiroga J, et al. Andrographolide, an Anti-Inflammatory Multitarget Drug: All Roads Lead to Cellular Metabolism. Molecules 2021; 26(1): 5.
- Li J, Huang L, He Z, et al. Andrographolide Suppresses the Growth and Metastasis of Luminal-Like Breast Cancer by Inhibiting the NF-κB/miR-21-5p/PDCD4 Signaling Pathway. Frontiers in Cell and Developmental Biology 2021; 9: 643525.
- Othman NS, Mohd Azman DK. Andrographolide Induces G2/M Cell Cycle Arrest and Apoptosis in Human Glioblastoma DBTRG-05MG Cell Line via ERK1/2 /c-Myc/p53 Signaling Pathway. Molecules 2022; 27(19): 6686.
- Chen Z, Tang WJ, Zhou YH, et al. Andrographolide inhibits non-small cell lung cancer cell proliferation through the activation of the mitochondrial apoptosis pathway and by reprogramming host glucose metabolism. Annals of Translational Medicine 2021; 9(22): 1701.
- Wang S, Li H, Chen S, et al. Andrographolide induces apoptosis in human osteosarcoma cells via the ROS/JNK pathway. International Journal of Oncology 2020; 56(6): 1417–1428.
- Lim SC, Jeon HJ, Kee KH, et al. Andrographolide induces apoptotic and non-apoptotic death and enhances tumor necrosis factor-related apoptosis-inducing ligand-mediated apoptosis in gastric cancer cells. Oncology Letters 2017; 13(5): 3837–3844.
- Nishida N, Yano H, Nishida T, et al. Angiogenesis in Cancer. Vascular Health and Risk Management 2006;2(3): 213–219.
- Duan MX, Zhou H, Wu QQ, et al. Andrographolide Protects against HG-Induced Inflammation, Apoptosis, Migration, and Impairment of Angiogenesis via PI3K/AKT-eNOS Signalling in HUVECs. Mediators of Inflammation 2019; 2019: 6168340.
- Derbyshire EJ, Calder PC. Respiratory Tract Infections and Antibiotic Resistance: A Protective Role for Vitamin D? Frontiers in Nutrition 2021; 8: 652469.
- Rajanna M, Bharathi B, Shivakumar BR, et al. Immunomodulatory effects of Andrographis paniculata extract in healthy adults – An open-label study. Journal of Ayurveda and Integrative Medicine 2021; 12(3): 529–534.
- Adiguna SP, Panggabean JA, Atikana A, et al. Antiviral Activities of Andrographolide and Its Derivatives: Mechanism of Action and Delivery System. Pharmaceuticals 2021; 14(11): 1102.
- Murgia V, Manti S, Licari A, et al. Upper Respiratory Tract Infection-Associated Acute Cough and the Urge to Cough: New Insights for Clinical Practice. Pediatric Allergy, Immunology, and Pulmonology 2020; 33(1): 3–11.
- Gupta S, Mishra KP, Ganju L. Broad-spectrum antiviral properties of andrographolide. Archives of Virology 2017; 162(3): 611–623.
- Banerjee M, Parai D, Chattopadhyay S, et al. Andrographolide: antibacterial activity against common bacteria of human health concern and possible mechanism of action. Folia Microbiologica 2017; 62(3): 237–244.
- Aldurrah Z, Kauli FSM, Rahim NA, et al. Antidepressant evaluation of Andrographis paniculata Nees extract and andrographolide in chronic unpredictable stress zebrafish model. Comparative Biochemistry and Physiology Part C: Toxicology & Pharmacology 2023; 271: 109678.
- Indirapriyadarshini R, Kanimozhi G, Natarajan D, et al. Andrographolide protects acute ultraviolet-B radiation-induced photodamages in the mouse skin. Archives of Dermatological Research 2023; 315(5): 1197–1205.
- Yang ES, Do Y, Cheon SY, et al. Andrographolide suppresses aerobic glycolysis and induces apoptotic cell death by inhibiting pyruvate dehydrogenase kinase 1 expression. Oncology Reports 2023; 49(4): 72.
- Carretta MD, Alarcón P, Jara E, et al. Andrographolide reduces IL-2 production in T-cells by interfering with NFAT and MAPK activation. European Journal of Pharmacology 2009; 602(2–3): 413–421.
- Tarigan TJE, Purwaningsih EH, Yusra, et al. Effects of Sambiloto (Andrographis paniculata) on GLP-1 and DPP-4 Concentrations between Normal and Prediabetic Subjects: A Crossover Study. Evidence-Based Complementary and Alternative Medicine 2022; 2022: 1535703.
- JC B, Baumgartner M, Palma R, et al. Andrographis paniculata decreases fatigue in patients with relapsing-remitting multiple sclerosis: a 12-month double-blind placebo-controlled pilot study. BMC Neurology 2016; 16: 77.
- Dimpfel W, Schombert L, Keplinger-Dimpfel IK, et al. Effects of an Adaptogenic Extract on Electrical Activity of the Brain in Elderly Subjects with Mild Cognitive Impairment: A Randomized, Double-Blind, Placebo-Controlled, Two-Armed Cross-Over Study. Pharmaceuticals 2020; 13(3): 45.
- Shang Y, Shen C, Stub T, et al. Adverse Effects of Andrographolide Derivative Medications Compared to the Safe use of Herbal Preparations of Andrographis paniculata: Results of a Systematic Review and Meta-Analysis of Clinical Studies. Frontiers in Pharmacology 2022; 13: 773282.
- Greco R, Siani F, Demartini C, et al. Andrographis Paniculata shows anti-nociceptive effects in an animal model of sensory hypersensitivity associated with migraine. Functional Neurology 2016; 31(1): 53–60.
- Sattayasai J, Srisuwan S, Arkaravichien T, et al. Effects of andrographolide on sexual functions, vascular reactivity and serum testosterone level in rodents. Food and Chemical Toxicology 2010; 48(7): 1934–1938.
- Zhang WX, Zhang ZM, Zhang ZQ, et al. Andrographolide induced acute kidney injury: Analysis of 26 cases reported in Chinese Literature. Nephrology 2014; 19(1): 21–26.
- Bothiraja C, Pawar AP, Shende VS, et al. Acute and subacute toxicity study of andrographolide bioactive in rodents: Evidence for the medicinal use as an alternative medicine. Comparative Clinical Pathology 2013; 22(6): 1123–1128.
- Suebsasana S, Pongnaratorn P, Sattayasai J, et al. Analgesic, antipyretic, anti-inflammatory and toxic effects of andrographolide derivatives in experimental animals. Archives of Pharmacal Research 2009; 32(9): 1191–1200.
- Huang H, Cao H, Xing C, et al. Andrographolide induce human embryonic stem cell apoptosis by oxidative stress response. Molecular & Cellular Toxicology 2019; 15(2): 209–219.
- Akbarsha MA, Murugaian P. Aspects of the male reproductive toxicity/male antifertility property of andrographolide in albino rats: effect on the testis and the cauda epididymidal spermatozoa. Phytotherapy Research 2000; 14(6): 432–435.
- Zhang WX, Zhang ZM, Zhang ZQ, et al. Andrographolide induced acute kidney injury: Analysis of 26 cases reported in Chinese Literature: Andrographolide induced AKI in Chinese Literature. Nephrology 2014; 19(1): 21–26.
- Xu J, Xing W, Yuan T, et al. Metabolic changes in the urine of andrographolide sodium bisulfite-treated rats. Human & Experimental Toxicology 2016; 35(2): 162–169.
- Sa-ngiamsuntorn K, Suksatu A, Pewkliang Y, et al. Anti-SARS-CoV-2 Activity of Andrographis paniculata Extract and Its Major Component Andrographolide in Human Lung Epithelial Cells and Cytotoxicity Evaluation in Major Organ Cell Representatives. Journal of Natural Products 2021; 84(4): 1261–1270.
- Al Batran R, Al-Bayaty F, Al-Obaidi MMJ, et al. Acute Toxicity and the Effect of Andrographolide on Porphyromonas gingivalis-Induced Hyperlipidemia in Rats. BioMed Research International 2013; 2013: 594012.
- Worakunphanich W, Thavorncharoensap M, Youngkong S, et al. Safety of Andrographis paniculata: A systematic review and meta-analysis. Pharmacoepidemiology and Drug Safety 2021; 30(6): 727–739.
- Kaewdech A, Nawalerspanya S, Assawasuwannakit S, et al. The use of Andrographis paniculata and its effects on liver biochemistry of patients with gastrointestinal problems in Thailand during the COVID-19 pandemic: a cross sectional study. Scientific Reports 2022; 12(1): 18213.